
Science in Christian
Perspective
Inverted Human Eye a Poor Design?
Jerry Bergman*
Northwest State
College
22-600 State Rt. 34
Archbold, OH 53402-9542
jdbrg@bright.net
From Perspectives on Science and Christian Faith 52 (March 2000): 18-30. Response: Lathi
© 2000 by the American Scientific Affiliation
It is often claimed that the human retina is poorly designed because it appears to be placed in the eye backwards. Its design, therefore, requires that light travel through the nerves and blood vessels to reach the photoreceptor cells located behind the eye’s wiring. We now know that specific functional reasons exist for this so-called backward placement of the photoreceptors. A major reason for the retina reversal is that it allows the rods and cones to interact with the retinal pigment epithelial cells that provide nutrients to the retina, recycle photopigments, provide an opaque layer to absorb excessive light, and perform other functions. This design is superior to other systems, because it allows close association with the pigmented epithelium required to maintain the photoreceptors. It is also critical in both the development and normal function of the retina.
A major argument for the existence of a Creator is called the Argument from Design. Proponents claim that the design existing in creation proves the existence of an Intelligent Designer. Darwinists try to disprove this observation by providing examples of what they claim is poor design in order to demonstrate that the natural world is in fact not designed, but is the result of blind, natural, impersonal forces. This view is called the blind watchmaker thesis by Dawkins.1 One of the most common examples of poor design used by Darwinists is the human retina. A common claim made in both the popular and scientific literature to support the blind watchmaker thesis is that the vertebrate eye is functionally sub- optimal because the retina photoreceptors are oriented away from incoming light.2 Dawkins explains why he considers this an example of poor design:
Any engineer would naturally assume that the photocells would point towards the light, with their wires leading backwards towards the brain. He would laugh at any suggestion that the photocells might point away from the light, with their wires departing on the side nearest the light. Yet this is exactly what happens in all vertebrate retinas. Each photocell is, in effect, wired in backwards, with its wire sticking out on the side nearest the light. The wire has to travel over the surface of the retina, to a point where it dives through a hole in the retina (the so-called ‘blind spot’) to join the optic nerve. This means that the light, instead of being granted an unrestricted passage to the photocells, has to pass through a forest of connecting wires, presumably suffering at least some attenuation and distortion (actually probably not much but, still, it is the principle of the thing that would offend any tidy-minded engineer!). I don’t know the exact explanation for this strange state of affairs. The relevant period of evolution is so long ago.3
Williams claimed the retina is not just an example, but one of the best examples of “poor design” in vertebrates, proving that the blind watchmaker, not an intelligent creator, created life. He notes:
… Every organism shows features that are functionally arbitrary or even maladaptive … My chosen classic is the vertebrate eye. It was used by Paley as a particularly forceful part of his theological argument from design. As he claimed, the eye is surely a superbly fashioned optical instrument. It is also something else, a superb example of maladaptive historical legacy. The retina consists of a series of special layers in the functionally appropriate sequence. A layer of light-sensitive cells (rods and cones) stimulate nerve endings from one or more layers of ganglion cells that carry out initial stages of information processing. From these ganglia, nerve fibers converge to form the main trunk of the optic nerve, which conveys the information to the brain. All layers are served by blood capillaries that provide their metabolic requirements. Unfortunately for Paley’s argument, the retina is upside down. The rods and cones are the bottom layer, and light reaches them only after passing through the nerves and blood vessels (emphasis mine).4
Williams admits that the vertebrate eye still functions very well despite the backward retina design, but argues that this fact does not negate his basic argument. He says:
… the fact of maladaptive design, however minimal in effect, spoils Paley’s argument that the eye shows intelligent prior planning, and the visual effect is real and routinely demonstrable.5
This topic is of great interest to creationists. As Diamond notes:
[of all of our features] none is more often cited by creationists in their attempts to refute natural selection than the human eye. In their opinion, so complex and perfect an organ could only have been created by design. Yet while it’s true that our eyes serve us well, we would see even better if they weren’t flawed by some bad design. Like other cells in our bodies, the retina’s photoreceptor cells are linked to a network of blood vessels and nerves. However, the vessels and nerves aren’t located behind the photo- receptors, where any sensible engineer would have placed them, but out in front of them, where they screen some of the incoming light. A camera designer who committed such a blunder would be fired immediately. By contrast, the eyes of the lowly squid, with the nerves artfully hidden behind the photoreceptors, are an example of design perfection. If the Creator had indeed lavished his best design on the creature he shaped in his own image, creationists would surely have to conclude that God is really a squid.6
Thwaites argues that the inverted retina problem hits at the core of the design argument, and that historically the design argument was a major basis of theism. He says:
Another example straight out of creationist tracts involves the vertebrate eye that humans must share with the other vertebrates … the vertebrate eye shows poor design when compared to the eye evolved by the cephalopods. The vertebrate eye has a blind spot where the retinal nerves and the blood vessels exit the eye. There is no comparable blind spot in the cephalopod eye. The structures of the retinas spell the difference. Everything a vertebrate sees is seen through the nerves and blood vessels of the retina since the photosensitive elements of the retina are on the far side of the retina away from the light source. Clearly the cephalopod solution to retinal structure is more logical, for they have the photosensitive elements of the retina facing the light. Certainly the creationists need to explain why we got the inferior design. I had thought that people were supposed to be the Creator’s chosen organism.7
Williams adds that “our eyes, and those of all other vertebrates, have the functionally stupid upside-down orientation of the retina” and that the “functionally sensible arrangement is in fact what is found in the eye of a squid and other mollusks.”8 An evaluation of this argument reveals it is not only naive but grossly erroneous.
The so-called inversion of the retina is considered a suboptimal design primarily due to its simplistic comparison with a camera. In Diamond’s words, “placing the rods and cones at the bottom layer and requiring light to pass through the nerves and blood vessels is the opposite of how a sensible engineer would have designed the eye.” He adds “a camera designer who committed such a blunder would be fired immediately.”9 And Edinger concluded: “The vertebrate eye is like a camera with the film loaded backward … if an engineer at Nikon designed a camera like that, he would be fired.”10 This conclusion is based not only on the assumption that placing nerves and blood vessels in front of the retina reduces the retina’s overall effectiveness but that another design as a whole would be superior.
Retina photoreceptors that face the eye lens are called verted, and photoreceptors that face the back of the eye are called inverted.11 Verted eyes are wired so the photoreceptors face toward the light and the nerves are placed behind the photoreceptor layer.12 Most invertebrates possess a verted-type eye; most vertebrates (including mammals, birds, amphibians and fish) possess an inverted-type eye. Most verted eye types are very simple, although a few types, such as the cephalopod eye (squids and octopus), are almost as complex as the vertebrate eye. Verted eyes tend to be functionally inferior, a conclusion usually determined by measuring performance in response to visual stimuli. Even the better verted eyes are still “overall quite inferior to the vertebrate eye.”13
In contrast to the claims of Dawkins et al., no evidence exists to support the claim that even the most advanced verted eye is superior to the inverted eye.
The most advanced invertebrate eye is that of certain cephalopods.14 The major anatomical difference between the human eye and the advanced cephalopod eye, such as the octopus, is the retina, which is not only verted but also lacks a fovea centralis. As an underwater animal which usually lives on the ocean bottom, the eye of the octopus is designed to detect motion, not detail as is true of human eyes, and must maximize its utilization of light since the ocean usually has little or no light at its lower depths. Barnes notes:
The cephalopod eye undoubtedly forms an image, but the animal’s visual perception is certainly quite different from that of man, which is greatly dependent upon interpretation by the brain. The cephalopod optic connections appear to be especially adapted for analyzing vertical and horizontal projections of objects in the visual field.15
The visual system used by cephalopods is poorly understood partly because understanding it is not a funding priority and partly because it is so complex. Meglitsch notes: “The cephalopods have the most highly developed nervous systems to be found in invertebrates, and correspondingly complex behavior patterns.”16
In contrast to the claims of Dawkins et al., no evidence exists to support the claim that even the most advanced verted eye is superior to the inverted eye. As Ayoub asks:
[Would] “hundreds of thousands of vertebrate species—in a great variety of terrestrial, marine and aerial environments—really see better with a visual system used by a handful of exclusive marine vertebrates? In the absence of any rigorous comparative evidence all claims that the cephalopod retina is functionally superior to the vertebrate retina remains entirely conjectural.”17
Judging by physiology, the verted cephalopod retina is clearly inferior to the inverted retina. Wells notes:
Compared with the vertebrate retina, the retina of Octopus is very simple. There are no equivalents of amacrine, bipolar or ganglion cells in the cephalopod; peripheral processing of the visual input must be much simpler.18
The octopus eye also contains a complex nerve plexus posterior to the receptors.19 Wells adds that the optic lobes must assume many of the functions of the inverted retina in vertebrates so that the “apparent relative simplicity of the cephalopod system is an illusion. It is a matter of stacking; amacrines, bipolars and ganglion cells are all there, but stuck onto the outer layer of the optic lobe rather than onto the back of the retina.”20
Pechenik indicates that although cephalopods can perceive shape, light intensity, and texture, they lack many of the advantages of an inverted retina, such as the ability to perceive small details.21 The visual system of the cephalopods is designed very differently than the inverted eye in other ways to enable them to function in their dark, water world. They can see only in black and white and have a narrow range of vision compared to humans. Their photoreceptor cell population is composed of only rods, and they contain a mere twenty million retina receptor cells compared to 126 million in humans.22 The rod’s outer segments contain rhodopsin pigment that has a maximum absorption in the blue-green part of the spectrum (475 nm), which is the predominant color in their environment. Photons change the rhodopsin to metarhodopsin and no further breakdown nor bleaching occurs.23
A second pigment in the octopus, retinochrome, has an absorption maximum of 490 nm, which is more sensitive to dim light. It evidently serves a supplementary role in the octopus vision system.24 Humans have one rod type and three cone types. One cone type has a light frequency of 430 nm (blue), another 530 nm (green), and the other 569 nm (red). Further, in bright light the cephalopod’s pupils become thin and slit-shaped, and are held in a horizontal position by an organ called a statocyst that uses gravity to determine the horizontal.25 Evidently they scan a thin but wide area for information, indicating that their visual world is considerably different from that of humans.26
Grzimek notes that their visual process is “quite similar to that of the batrachians, reptiles and insects. A ‘photograph’ of the recorded image is not traced on the retina as in man; instead cephalopods record and interpret as stimuli (pattern recognition ) only light and color variations of a moving object.”27 Importantly, the octopus “will respond to certain motions as if they were prey, but will not react to his normal food-objects when they are motionless.”28 This observation of the importance of motion in vision function is in harmony with the observation that the octopus eye can be called “a compound eye with a single lens” for the reason that the receptor cells are surrounded by microvilli which form rhabdomeres.29 Each facet in a compound eye is either on or off, and object movement produces a change in the on-and-off pattern, similar to how a series of light bulbs produces the illusion of movement by changing on-and-off patterns.
How the eye
evolved from the primitive verted type common
to invertebrates into the inverted eye of vertebrates is … an unexplained mystery. No
evidence exists of any transitional forms, and all known animals have either verted or
inverted eyes.
Our ignorance about the function of major parts of the cephalopod visual system, such as the optic lobe, prevents researchers from completing a more detailed analysis of cephalopod vision. How the eye evolved from the primitive verted type common to invertebrates into the inverted eye of vertebrates is also so far an unexplained mystery. No evidence exists of any transitional forms, and all known animals have either verted or inverted eyes. Prince notes:
[one of the essential and] most important differences between vertebrate and invertebrate eyes is that in the former the receptors point outwards towards the choroid, whereas in invertebrates they mostly point inwards towards the lens. But for that obstacle we should have been deluged with theories on the original evolution of the vertebrate eye from the invertebrate. As it is, vertebrate visual origins have to be approached with great caution, and … there is nothing indisputable which can be used to explain the origins of the vertebrate eye from an invertebrate organ.30
The common solution, convergent evolution, suffers from major problems and will be discussed elsewhere.
Functions of Rods and Cones in Vertebrates
To understand the critical function of the retinal pigment epithelium (RPE), the chemical process required for vision must be briefly summarized. The rods and cones are photoreceptor cells located in the retina that transduce light into electrical signals. Rods and cones are cells that contain most of the organelles that cells normally require, including mitochondria, Golgi complexes, a nucleus etc. So-called black-and-white transduction occurs in the rod-shaped receptors, and color transduction occurs largely in the cone shaped receptors.31
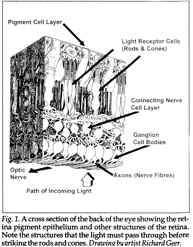
The inverted retina vision system requires light to first pass through the cornea, then through the anterior chamber filled with aqueous fluid, then through the lens, and then through the vitreous humor fluid. Finally, before reaching the retina, light passes through the inner cell layers of the retina, past the rod and cone photoreceptors, until it reaches the far posterior or distal end of these cells, wherein lie the so called outer cell segments. See Fig. 1.
The outer segment membrane in cones folds back and forth in a pleated fashion, and in rods the pleats pinch off to form close to 1,000 separate disks piled up like neatly stacked pennies. The outer cell segments contain the photoreceptor light-sensitive structures including the visual pigment, also called the photopigment. The photopigment is where the transduction of light into receptor potentials occurs.
The photopigments consist of a family of proteins that undergo physical changes when they absorb light energy. The principal component of photopigments is the opsin glycoprotein, a derivative of retinal (a modified vitamin A molecule). Vitamin A is derived from carotenoids. For this reason, good vision requires a diet high in foods that contain abundant carotenoids, such as carrots, spinach, and broccoli. Lack of vitamin A produces night blindness or nyctalopia. Rods contain a single photopigment type called rhodopsin (rhodo meaning rose and opsis meaning vision). The cones contain one of three different kinds of photopigments called iodopsins: (1) erythrolabe (which is most sensitive to red light), (2) chlorolabe (most sensitive to green light), and (3) cyanolabe (most sensitive to blue light).32 Color vision occurs due to small variations in the amino acid sequences of these different iodopsins, which enable the rods and cones to differentially absorb wavelengths of incoming light.
Vision functions by a change in the retina photopigments molecule caused by light. The molecule has a bent shape (cis-retinal) in darkness, and when it absorbs light, isomerization occurs and the molecule becomes the ‘straight’ form (trans-retinal). This causes several unstable intermediate chemicals to form, and after about one minute, trans-retinal completely separates from opsin, causing the photopigment to appear colorless (for this reason, the process is called bleaching). So that the disk rods or cones can again function for vision, retinal must be converted from trans back to the cis form. This resynthesis process called regeneration is aided by the pigment epithelium cells located next to the rod and cone outer segments.
Vision functions by a change in the retina photopigments molecule caused by light.
Cone photopigments regenerate more quickly than do the rhodopsins and consequently “are less dependent on the pigment epithelium.”33 A half-life of about five minutes is required for rhodopsin regeneration in the rods compared to 1.5 minutes for iodopsin regeneration in cones. Excessive light will cause blindness in the effected rods and cones until this regeneration process occurs. Common examples of this phenomena include temporary blindness after watching a very bright light flash, such as from a strobe-light or photocopy machine.34
When rods and cones are stimulated by light, they release neurotransmitters that induce graded, local potentials in both bipolar and horizontal cells. By this means, the rod and cone outer segments transduce light into electrical signals. The signals are carried by the central nervous system neurons to bipolar cells, which in turn synapse with the ganglion cells, then to the lateral geniculate body of the thalamus and other centers in the brain stem, and, lastly, to the primary visual center in the occipital lobe of the cortex where the signals are interpreted by the brain.
The first level of processing visual information actually occurs by the amacrine cells, which transmit information between adjacent bipolar cells and ganglion cells, allowing lateral communication in the outer retina for a comparison of information. Retinal amacrine cells help to process visual information by enhancing certain aspects and discarding other aspects of visual input. Input from several cells can either converge upon one postsynaptic neuron or diverge to several post synaptic neurons. Convergence dominates to the degree that in humans about 126 million photoreceptors send their information to only about one million ganglion cells.
A single cone tends to synapse with one bipolar cell whereas between six and 600 rods synapse with a single bipolar cell. The cones’ one-to-one synapses give them much higher visual acuity but lower sensitivity. In contrast, rods are extremely sensitive to light but their visual acuity is not as sharp. Horizontal cells transmit inhibitory signals to the bipolar cells, which enhance the contrast between areas of the retina that are strongly stimulated and adjacent areas that are more weakly stimulated. Lastly, ganglion cell axons that form the optic nerve carry the information to the brain for processing.
The Function of the Retinal Pigment Epithelium
As noted, vision depends upon the isomerization of 11-cis-retinal to 11-trans-retinal. Each light photon striking a photoreceptor isomerizes retinal, and billions of photons can strike the retina at any one second. The 11-cis-retinal must be regularly replaced to maintain the cycle, a task for which the retinal pigment epithelium (RPE) is critical. RPE is a single cell layer thick, consisting of relatively uniform cells whose apical end is covered with dense microvilli. RPE cells are polygonally shaped and contain apical microvilli and basal membrane enfoldings. Their tight junction cell connections help to seal the vitreous humor in the eyeball and contribute to the blood-retina barrier. Posterior to the RPE is the vascular choroid layer, and posterior to that is the connective tissue known as the sclera.
Research on the
eyes of different species has found that,
although major differences
among them exist,
the retinal pigment epithelium
shows “little variation.”
RPE touches the extremities of the photoreceptors, both the rods and the cones, and the microvilli interdigitate with their sides.35
The interphotoreceptor matrix contains soluble and insoluble components which are critical to the photoreceptor/RPE functions. Seemingly simple in appearance, the RPE has “a complex structural and functional polarity that allows them to perform highly specialized roles.”36 One of their major functions is to collect the used retinal from the photoreceptors. They then use vitamin A to regenerate the retinal, after which it is transferred back to the photoreceptors.37
Vitamin A regeneration requires the RPE to manufacture retinol isomerase and other compounds. The RPE also stores large quantities of vitamin A.
Research on the eyes of different species has found that, although major differences among them exist, the RPE shows “little variation.”38
The small variations that exist in the RPE are due to differences in the retina structure, indicating its critical role in the vision of all vertebrates. One study found retinol isomerase existed in all the major vertebrates tested and was lacking in all three cephalopods tested.39 Bridges concluded that reciprocal flow of retinoids between the retina and the site of isomerase action in the RPE is a feature common to the visual cycle in all vertebrates.40 Since RPE cells use much energy and nutrients, they must be in intimate contact with both the photoreceptors and the blood supply, in this case the choroid, to carry out this critical function.41
Phagocytic Role of the Retinal Pigment Epithelium
Another role of the RPE is to recycle the used rod and cone outer segment membranes, the portion closest to the RPE. This area is often referred to as the business end of the photoreceptor cells, because it is here where the membranous disks that respond to light are located. Cones usually contain from 1,000 to 1,200 disks, and rods from 700 to 1,000. The high level of outer segment activity requires them to be continually replaced.42 New outer segment membranes continually grow at the outer photoreceptor segment base, adding to the photoreceptor length.
Photoreceptor outer segments are renewed at “an astonishingly rapid pace.”43 A normal rod photoreceptor sheds about 10% of its outer segment disks at its apex and renews the same amount daily.44 As the outer segment lengthens from its base, the oldest membrane, which is the distal end, is shed in segments of one to three disks at a time. Those sloughed off are phagocytized by the RPE in order to recycle its parts.45 This process is continuous, effectively maintaining the high sensitivity of the photoreceptors.46 A summary of this cycle by Bok and Young is as follows:
… the retinal pigment epithelium carries out several functions that are crucial for the normal operation of the visual system. One of these important roles, appreciated for about a decade, is the phagocytosis of rod outer segment debris. This scavenging activity goes on daily at an impressive rate in the normal retina. It can be accelerated to extraordinary levels when outer segments are damaged. Disruption of this phagocytic function may underlie a variety of clinical disorders, some of which result in blindness.47
RPE microvilli interdigitate and surround the photoreceptor outer segments to effectively carry out their phagocytic and recycling role. The first step in phagocytosis is recognition (causing their binding to the RPE apical microvilli) followed by invagination and, lastly, ingestion by phagosomes. Pseudopods that engulf the rod and cone outer receptor fragments are controlled by actin in much the same way that single-celled animals, such as the amoeba, use pseudopods to consume food. The RPE then breaks down the ingested material by enzymes stored in its lysosomes. Lastly, the free radicals and superoxides produced by enzyme action in the RPE must be neutralized by superoxide dismutase, peroxidase, and other enzymes.48
Nutrient Role of the Retinal Pigment Epithelium
The RPE selectively transports nutrients from choroidal circulation to both the photoreceptors and retinal cells. The RPE cell-tight junctions prevent diffusion of even small molecules into the vitreous humor and insures that the metabolites required by the outer retina can move to where they are needed.49 The RPE has a function like that of a placenta to insure that the outer retina is protected from injurious compounds and yet allows the necessary nutrients to pass into the area of the rods and cones. To insure that enough of the needed nutrients pass the RPE barrier, the basal membrane is highly enfolded to produce more surface area. This role is critical because the rods and cones require a greater blood supply than any other bodily tissue.50 Which compounds pass though are determined by basal membrane receptors. RPE also synthesizes and secretes extracellular matrix molecules.
Processing Visual Information
The potential interference of light as it traverses several layers of retina before reaching the photoreceptors in an inverted eye is overcome by visual processing. When bipolar or amacrine cells transmit excitatory signals to ganglion cells, the ganglion cells become depolarized, initiating a nerve impulse. Nerve impulses travel along axons of the optic (II) cranial nerve, leading to the optic chiasm where some fibers cross over to the opposite side and some remain on the same side (chiasma means cross as shown by the letter X). On the other side of the optic chiasm, the fibers are named the optic tract and synapse with neurons in the lateral geniculate nucleus of the thalamus. The lateral geniculate nucleus neurons then form a passageway called the optic radiations to carry the information for processing to the primary visual areas in the occipital lobes of the cerebral cortex.
Each eye sees a slightly different visual field, and the large overlapping area is called the binocular visual field used to produce stereo vision. One eye will see a crescent-shaped peripheral monocular visual field that the other eye cannot see, and the same will occur on the opposite side with the opposite eye. Also, each eye has a blind spot caused by a hole in the retina where the optic nerve must pass through in order to travel to the brain. This blind spot falls on a different place in each retina, and the information from both eyes is combined so that these visual blind spots are not normally perceived.
The potential interference of light as it traverses several layers of retina before reaching the photoreceptors in an inverted eye is overcome by visual processing.
Light rays from an object in the temporal half of the visual field (that facing away from the nose) will fall in the nasal half of the retina, and conversely light rays from an object in the nasal half of the visual field will fall on the temporal half of the retina, reversing the image as occurs when a transparent slide is projected by a slide projector. Also, light rays at the top of the visual field strike the inferior portion of the retina, and those at the bottom of the visual field are projected on the superior portion of the retina, again reversing the image. Both the left-right and up-down reversal must be corrected by the brain.
Information received by the brain must be extensively processed in other ways as well. This complex operation involves at least three separate systems located in the cerebral cortex, each with a specific function. One system processes information related to shape, another regarding color, and a third about movement, location, and spatial organization of the object. Goldsmith concluded that the optical design of the vertebrate eye “approaches optima predicted from physics” and that in the real world:
animals have a way of confounding the assumptions and boundary conditions in hypothesized models of optimal behavior. In dealing with the interrelated sensory tasks of maximizing spatial acuity and contrast sensitivity, however, both the “camera” eyes of Old World primates and birds, as well as the compound eyes of diurnal insects, present clear examples of evolutionary optimization … The investigator’s task in examining the hypothesis of optimization is therefore to ask how closely the optical performance of eyes of different optical design approaches the limits set by physics … Despite the very different modes of design that underlie the construction of the single-lens eyes of vertebrates and the compound eyes of arthropods, similar considerations determine their capacities to resolve images.51
The Macula
An area of the retina in the central macula called the central fovea is part of the solution to the problem of light loss due to the reversed retina. The nerve cell bodies in this area are displaced sideways to provide a clearer path for light to reach the photoreceptor cells.52 The macula area is no larger than pencil lead in diameter but is about 100 times more sensitive to small features than the rest of the retina. Vision is the sharpest at the macula, which is critical in providing the brain with information needed to construct an image. It allows us to read, watch television, recognize friends, and even walk. Most of the rest of the retina actually is concerned with peripheral vision. The macula provides information needed to maximize image detail, and the information obtained by the peripheral areas of the retina helps to provide both spatial and contextual information.
The peripheral retina also functions to survey a large visual area for clues to determine where a person should focus his or her macula for more input. The peripheral area does not need to pick up much detail because its role is primarily to inform the brain of locations that may need more informational input. This structure allows the person to be aware of a wide visual field, yet at the same time not be distracted by it.
An area of the
retina in the central macula called the central fovea
is part of the solution to the problem of light loss
due to the reversed retina.
If the entire retina were sensitive to the same level of detail as the macula, the brain would suffer from sensory overload and not function properly. The sensory overload problem is well understood from research on hyperactivity and auditory sensory overload. If the retina were reversed so that the rods and cones faced in the direction of the light, the peripheral area may require a means of lowering the light intensity.
The importance of the RPE is indicated by the fact that one of the most common causes of blindness in the developed world, macular degeneration, is a result of RPE deterioration.53 In this disease, the eye’s macula loses its ability to function, causing major central vision loss. In macular degeneration, not only does the central vision deteriorate but the patient is less able to focus on an object of interest. The retinal pigment cells do not replace themselves by cell division as do most cells. Consequently, when they are damaged, the retina cells also soon die. Demise of the RPE is often caused by intracellular accumulation of excessive levels of lipofuscin damaged so severely that the cell’s native enzymes cannot properly degrade them.54 Central serous retinopathy is also considered to be a RPE disorder, specifically its ion pump function, and/or a result of choroidal vascular hyperpermeability.
Detached Retina and the Role of Pigment Epithelial Cells
The retina is evidently held to the RPE largely by the interphotoreceptor matrix. When the retina pulls away from the pigment epithelium at the interphotoreceptor matrix area, a detached retina results. Fluid that accumulates between the neuron portion of the retina and the pigment epithelium gradually forces the thin pliable retina to billow out toward the lens of the eye. Some results of this change are visual field defects, light flashes, floaters, and distorted vision caused by optical effects resulting from the new position of the retina in relationship to the lens.55
Detachment of the retina from the pigment epithelium also causes a drastic reduction in the rhodopsin regeneration rate. As a result, when the pigment epithelium can no longer function to regenerate the rods and cones, vision is distorted. Eventually the death of significant amounts of retina tissue occurs in those areas that have become detached from the RPE. The retinal detachment can sometimes be halted by migrating pigment epithelium cells that bond to the separating retina, preventing its progressive separation. When this occurs a scar is formed on the retina called a pigmentation line. If this system fails, the progressive detachment can often be halted by laser therapy, a procedure only minimally invasive, because laser light is able to pass through the cornea and the lens without damaging them. Laser therapy stimulates the migration of the pigment epithelium cells, inducing the pigmentation line.
Functions of the Pigment
The pigment epithelium sheet consists of epithelial cells that produce organelles containing melanin granules. Since RPE cells are located between the choroid and the retina, they often are classified as part of the choroid instead of the retina. The melanin they contain functions to absorb stray light, preventing the reflection and scattering of light within the eyeball, and ensuring that the image cast on the retina by the cornea and lens remains sharp and clear.
Another function of the pigment is to form an opaque screen behind the optical path of the photoreceptors. This light absorptive property of the pigment is critical to maintaining high visual acuity. Hewitt and Adler concluded that the diverse function of the retinal pigment epithelium cells “is essential for the normal functioning of the outer retina.”56 For this reason, normal retinal function requires that the RPE and photoreceptors be in close proximity. A summary of the role of the RPE is as follows:
The rods and cones are constantly replacing the visual pigment disks. The old ones are discarded toward the outside, where the pigment epithelium cells absorb them. Were the disks to be disposed of toward the incoming light, we would soon expect a murky situation inside the eye. The rods and cones take no vacation, the disks are constantly being replaced throughout our lifetime … The reason for renewal of the disks in the eye … [includes] preventive maintenance and a way of providing a fresh supply of visually sensitive chemicals. It appears that the disks [are] … absorbed at the end of the rods.
Tapetum Lucidus
Many animals contain a structure called a tapetum lucidus in addition to the pigmented epithelium. The layer called the tapetum effectively reflects the incoming light back to the rods, giving them a second opportunity to absorb light, thus providing much greater visual acuity in low light levels.58 The tapetum produces the reflective eyes characteristic of nocturnal animals.59 This structure gives cat, dog, and deep-sea or turbid-water fish eyes the distinctive glow at night called eyeshine, which causes them to appear to be lit up. Excellent night vision allows predators to prowl at night when competition for food and space is less. A cat’s night vision is estimated to be six times better than humans. Their eyes are so effective that they can operate in light that humans perceive as close to pitch black. The tapetum’s importance is indicated by the fact that the part of the pigment epithelium which covers the tapetum is “always devoid of pigment so that there is no interference with the back reflection of the light.”60
This structure allows a cat to see much better in dim light but at a cost of much poorer resolution and less visual clarity during daylight.61 The cat has more rods than cones compared to humans; consequently, it is more sensitive to low light, but has much less resolving power and an inferior ability to detect colors compared to humans. Animals with a tapetum usually have poor vision during daylight hours and many possess highly contractile pupils to protect their retina.62
The Retina Pigmented Epithelium’s Role in Development
Pigmented epithelium is also critical for normal vertebrate eye development. Raymond and Jackson conclude from their study:
… a series of reciprocal cellular interactions that determine the fate of the eye components … [exists during development and the] presence of the RPE is required for the normal development of the eye in vivo. Its presence early in development is necessary for the correct morphogenesis of the neural retina. After the neural retina has started to differentiate, the RPE is still necessary, either directly or indirectly, to maintain the organization of the retinal lamina.63
The RPE actually plays a succession of roles during embryonic development, including trophic influence, transport functions, retinomotor response, and phagocytic and inductive interaction.64
Other Possible Designs
If the human retina were verted, we have no evidence that vision would be better. Most likely it would be worse. Comparisons of different eyes are difficult to make because, although the quality of the image projected on the retina can be evaluated by a study of the lens system’s optical traits, we lack direct knowledge about the actual image produced in the brain.
A major concern, when critiquing the existing vertebrate retina design, involves speculations on the quality of vision that would result from another design. If the retina were reversed, the RPE or its analog and its cellular support system would have to be placed either in front of the photoreceptors or on their side. These approaches are clearly inferior to the existing vertebrate system that produces superior sight for terrestrial animals. If located in front of the retina, depending on how transparent those cells were, this design could prevent most light from reaching the photoreceptors.
If the RPE were located on each side of the rods and cones, as in the cephalopods, primarily only the front of the sensory cells would be able to respond to light. Prince even claims the cephalopods side design “is protective and shields the receptors from excess light.”65 Opaque wastes would accumulate in the path of light, and nutrients would have to be plentiful, thereby further diminishing the amount of light reaching the photoreceptors. Surrounding each photoreceptor RPE retina cell also requires increasing the space between the photoreceptors, further decreasing the amount of light able to strike the photoreceptors, consequently lowering vision resolution. Recycling of the outer segments so photoreceptors can be quickly regenerated would also be a problem, if the photoreceptors faced the vision light path line. If the eye were designed according to the Darwinist plan, the following would be the result:
Should the disk end of the rods and cones be reversed in direction so as to face the light, as some evolutionists suggest they should, we would probably have a visual disaster. What would perform the essential function of absorbing the some 10,000 million disks produced each day in each of our eyes? They would probably accumulate in the vitreous humor region and soon interfere with light en route to the retina. If the pigment epithelium layer were placed on the inside of the retina so as to absorb the disks, it would also interfere with light trying to reach the rods and cones. Furthermore, the pigment epithelium, which is closely associated with the disk ends of the rods and cones, also provides them with nutrients for making new disks. The epithelium gets its nutrients from the rich blood supply in the choroid layer next to it. In order for the pigment epithelium to function properly, it needs this blood supply. To put both the pigment epithelium and its choroid blood supply on the inside of the eye, between the light source and the light-sensitive rods and cones, would severely disrupt the visual process.66
The sensitivity of the existing human inverted design is so great that only one photon is able to elicit an electrical response.67 Consequently, functional sensitivity of the verted retina could not be significantly improved. Ferl and Wallace note:
Neurobiologists have yet to determine how such a negative system of operation might be adaptive, but they marvel over the acute sensitivity possible in rod cells. Apparently rod cells are excellent amplifiers. A single photon (unit of light) can produce a detectable electrical signal in the retina, and the human brain can actually “see” a cluster of five photons—a small point of light, indeed.68
Greater sensitivity of the inverted retina, if this were possible, may result in poorer vision due to sensory overload. Williams syndrome patients have hearing so superior to that of normal persons that they can hear a faint whisper. Unfortunately, this causes them serious problems dealing with loud noises; thunder is actually physically painful.
If the human retina were verted, we have no evidence that vision would be better. Most likely it would be worse.
Though higher visual acuity may improve night vision, it would surely result in difficultly seeing during daylight hours.69 This would not be functional for most people who must work in a normal human environment. Actually, a case can be made that more light blockage of the retina would be functional. Many people must wear sunglasses because the outdoor light is too bright. In a review of the literature, Young found that excess light is now a serious health problem. He notes:
All of the major cellular and molecular features of age-related cataract have been reproduced in the laboratory … solar radiation can with similar cogency explain the distinctive global pattern of age-related cataract among human populations—the risk of cataract depends on where one lives on the surface of the earth … When cataract blindness statistics from 55 different countries of the world are grouped according to latitude, it is found that in the tropics there is a fivefold increase in blindness resulting from cataract than at northern latitudes, whereas intermediate latitudes fall in between. Although many factors are involved, sunlight is the only one known to vary in a gradient from high in the tropics to low in the northern latitudes … Current evidence provides the basis for the design of protective lenses that minimize the hazards of sunlight exposure without significantly interfering with vision. The prescription has two components—one to protect the lens, the other to protect the retina … Use of sunglasses … should begin early in childhood and be continued throughout the life span whenever exposure to bright sunlight is desirable or necessary. Radiation damage to delicate ocular structures can occur at any age and tends to be cumulative. Even modestly effective preventive measures may produce highly significant benefits if applied over an extended period.70
Albinos lack pigment in their pigment epithelium cells, and consequently they often suffer from foveal hypoplasia. As a result, they lack the detailed central vision. They also lack iris pigment and must wear sunglasses because even moderately bright light may severely adversely affect their vision.71 Even blue-eyed people are at a disadvantage because the blue pigment allows more light in than darker iris colors. Consequently, blue-eyed people suffer from more vision problems and blindness.7272 Being able to effectively read with very dim light may be an improvement in some situations, but since most human activities occur during daylight hours, and darkness is functional to induce sleep due to pineal gland activity, the existing secretion system appears to be the most effective.
Furthermore, although the light yellow tint of the eye lens filters out some ultraviolet light, the inverted eye design serves to filter out much of the remaining ultraviolet light. The incoming light must pass through the overlying neural components and blood vessels and the penetrating power of ultraviolet light is markedly inferior to white light.73 The verted eye is used in animals, such as the octopus, that live under water where most of the ultraviolet light is filtered out. Consequently, they have less need for this protection.
Given the role of the pigmented epithelium, it is clear that the existing design is an ideal compromise. The main question is: “Is the retina reversal an obstacle to vision?” Williams notes that the vertebrate eye works quite effectively despite the retina reversal because it is a precise visual instrument designed to function with the rods and cones facing away from the light. He explains:
The tissues intervening between the transparent humors of the eye cavity and the optically sensitive layer are microscopically thin. The absorption and scatter of light is ordinarily minor, and functional impairment seldom serious … Red blood cells are poor transmitters of light, but when moving single file through capillaries can cause only a negligible shading of the light sensors. In larger venuoles and arterioles they cast dense shadows and blot out images. That we do not ordinarily perceive these shadows is the result of minute involuntary eye movements, which keep the blood-vessel shadows moving, and of our brains recording the flux of images as continuous pictures. The reality of the shadow of the vascular tree … can be demonstrated with a flashlight and instructions from a visual physiologist.74
Nerve cell fibers and the small branches of the central retina artery and vein actually produce minimal hindrance to light reaching the photoreceptors. Most cells are 60 to 70 percent water and thus are largely transparent. In contrast to most peripheral nerves, nerve fibers in front of the retina are not mylinated. Myelin, an opaque, whitish lipid that coats the nerves, would block much light. These facts have forced Dawkins to note:
With one exception, all the eyes I have so far illustrated have had their photocells in front of the nerves connecting them to the brain. This is the obvious way to do it, but it is not universal. The flatworm … keeps its photocells apparently on the wrong side of their connecting nerves. So does our own vertebrate eye. The photocells point backwards, away from the light. This is not as silly as it sounds. Since they are very tiny and transparent, it doesn’t much matter which way they point: most photons will go straight through and then run the gauntlet of pigment-laden baffles waiting to catch them.75
Moving shadows produced by the venules and arterioles are also highly functional because they produce momentary darkness to aid in the rod and cone regeneration. Constant bright light would excessively bleach the photopigment, and lower light achieved by the existing design allows their regeneration. Further, as noted above, the RPE metabolic machinery is “essential for the normal functioning of the outer retina [and] because of the nature of these interactions, it is essential that the RPE and photoreceptors be in close proximity” for normal retina function.76
Given the role of the pigmented epithelium, it is clear that the existing design is an ideal compromise.
The inverted eye also produces the most acute image of all known designs. The eyes of birds not only produce the sharpest vision of all known animals, but they can form sharp images on all areas of their inverted retina. In addition, they have two to five times the number of cones per square millimeter as do humans.77 Birds also rely on a large structure that protrudes into the retina called a pectin, which most likely replaces the embedded blood vessels in mammals. This system evidently interferes less with vision than would a network of blood vessels, and is another reason why birds have unusually high visual activity.78 Many reptiles have a structure similar to the pectin called a conus papillaris, which is not pleated, is more cone-like, and often differs in other ways from the pectin structure.79
Birds are also sensitive to light in the near ultraviolet spectrum, and have red oil droplets in the lower part of their eyeball cavity that enhances the contrast of objects, such as animals in a green foliage background. Furthermore, their eyeball contains yellow droplets in the upper area of the eyeball that enhances objects seen against the sky by filtering out much of the blue background. The two different oils are kept separate by density differences. These modifications help animals to see in their world but would be a major hindrance to humans in our terrestrial world.
Conclusions
Claims of poor retina design are often raised by evolutionists to argue against Intelligent Design.80 A review of research on the vertebrate retina indicates that for vertebrates the existing inverted design is superior to the verted design, even the system used by the most advanced cephalopods. Its design has been maximized for life in our environment and no doubt would function poorly in another environment, such as that experienced by undersea bottom dwellers. This review supports Hamilton’s conclusion:
Instead of being a great disadvantage, or a “curse” or being incorrectly constructed, the inverted retina is a tremendous advance in function and design compared with the simple and less complicated verted arrangement. One problem amongst many, for evolutionists, is to explain how this abrupt major retinal transformation from the verted type in invertebrates to the inverted vertebrate model came about as nothing in paleontology offers any support.81
Rather than being fired, our camera designer would no doubt be promoted for utilizing a less obvious, but, as a whole, a far more functional design.
Acknowledgments
I wish to thank Dr. Tara Richmond O.D. for her comments on an earlier draft of this manuscript.
©2000
1 Richard Dawkins, The Blind Watchmaker (New York: W. W. Norton, 1986).
2 George Ayoub, “On the Design of the Vertebrate Retina,” Origins and Design 17:1 (Winter 1996): 19.
3 Dawkins. The Blind Watchmaker, 93.
4 George C.Williams, Natural Selection: Domains, Levels, and Challenges (New York: Oxford University Press, 1992), 72.
5 Ibid., 73.
6 J. Diamond, “Voyage of the Overloaded Ark,” Discover (June 1985): 91.
7 William Thwaites, “Design, Can We See the Hand of Evolution in the Things it has Wrought?” Proceedings of the 63rd Annual Meeting of the Pacific Division American Association of the Advancement of Science 1:3 (1982): 210.
8 George C. Williams, The Pony Fish Glow and other Clues to Plan and Purpose in Nature (New York: Basic Books, 1997), 9–10.
9 Diamond, “Voyage of the Overloaded Ark,” 91.
10 Steve Edinger, “Is there a Scientific Basis for Creationism?” The Congressional Quarterly Researcher 7:32 (August 22, 1997): 761.
11 Santiago Cajal, The Structure of the Retina, (Springfield, IL: Charles C. Thomas, 1972).
12 Kenneth Miller, “Life’s Grand Design” Technology Review 97:1 (Feb, March 1994): 32.
13 H. S. Hamilton, “The Retina of the Eye—An Evolutionary Road Block,” CRSQ 22 (Sept. 1985): 60.
14 Joan Abbott, et al. Cephalopod Neurobiology 2d ed. (New York: Oxford University Press, 1995).
15 Robert D. Barnes, Invertebrate Zoology (Philadelphia, PA: Saunders, 1980), 454.
16 Paul Meglitsch, Invertebrate Zoology (New York: Oxford, 1972), 356.
17 Ayoub, “On the Design of the Vertebrate Retina,” 20.
18 Martin John Wells, Octopus: Physiology and Behavior of an Advanced Invertebrate (London: Chapman and Hall, 1978), 150.
19 Meglitsch, Invertebrate Zoology, 356.
20 Wells, Octopus: Physiology and Behavior of an Advanced Invertebrate, 150.
21 Jan Pechenik, Biology of the Invertebrates (Dubuque, IA: Wm. C. Brown, 1991), 312.
22 J. Z. Young, “The Anatomy of the Nervous System,” Octopus Vulgaris (New York: Oxford University Press, 1971), 441.
23 Wells, Octopus: Physiology and Behavior of an Advanced Invertebrate, 145.
24 Ibid., 146.
25 Young, “The Anatomy of the Nervous System.”
26 H. S. Hamilton, “Convergent evolution-Do the Octopus and Human eyes qualify?” CRSQ 24 (1987): 82–5.
27 Bernhard Grzimek, Grzimek’s Animal Life Encyclopedia (New York: Van Nostrand Reinhold Co., 1972), 191.
28 Irwin M. Spigel, ed., Readings in the Study of Visually Perceived Movement (New York: Harper & Row, 1965), 126.
29 B. V. Budelmann, “Cephalopod Sense Organs, Nerves and the Brain: Adaptations for high performance and life style,” in Physiology of Cephalopod Mollusks, ed. Hans Portner, et al. (Australia: Gordon and Breach Pub., 1994), 15.
30 Jack Prince, Comparative Anatomy of the Eye (Springfield: Charles Thomas, 1956), 334, 348.
31 Steven J. Ryan, ed., The Retina 2d ed. (St Louis: Mosby, 1994).
32 David Shier, Jackie Butler, and Ricki Lewis, Hole’s Human Anatomy and Physiology (Dubuque, IA: Wm.C. Brown Pub. Co., 1999), 482.
33 Gerald Tortora and Sandra Grabowski, Principles of Anatomy and Physiology (New York: Harper and Collins, 1996), 468.
34 Richard Snell and Michael Lemp, Clinical Anatomy of The Eye (Boston: Blackwell Scientific Pub., 1989).
35 Roy H. Steinberg and Irmgard Wood, “The Relationship of the Retinal Pigment Epithelium to the Photoreceptor Outer Segment in the Human Retina,” chap. 2 in The Retinnal Pigment Epithelium ed. Keith M. Zinn and Michael F. Marmor, (Cambridge; MA: Harvard University Press, 1994), 39.
36 A. T. Hewitt and Rubin Adler, “The Retinal Pigment Epithelium and Interphotoreceptor Matrix: Structure and Specialized Functions” in The Retina, 58.
37 Ibid.
38 Toichiro Kuwabara, “Species Differences in the Retinal Pigment Epithelium,” chap. 5 in The Retinal Pigment Epithelium, 58.
39 C. D. B. Bridges, “Distribution of Retinol Isomerase in Vertebrate Eyes and its Emergence During Retinal Development,” Vision Research 29:12 (1989): 1711–7.
40 Ibid., 1711.
41 George Marshall, “An Eye for an Eye: An Interview with Eye Disease Researcher Dr. George Marshall, University of Glasgow, Scotland, Creation Ex Nihilo 18:4 (1996): 19–21.
42 Dean Bok, “Retinal Photoreceptor disc shedding and pigment epithelium phagocytosis,” in The Retinal Pigment Epithelium, 148.
43 Tortora and Grabowski, Principles of Anatomy and Physiology, 467.
44 Eliot Benson, “Retinitis Pigmentosa: Unfolding its Mystery,” Proceedings of the National Academy of Science USA 93 (May 1996): 4526–28.
45 Tortora and Grabowski, Principles of Anatomy and Physiology, 467.
46 Benson, “Retinitis Pigmentosa: Unfolding its Mystery.”
47 Dean Bok and Richard Young, “Phagocytic Properties of the Retinal Pigment,” in The Retinal Pigment Epithelium, 148.
48 Hewitt and Adler, “The Retinal Pigment Epithelium and Interphotoreceptor Matrix: Structure and Specialized Functions,” 60.
49 Ibid., 59.
50 Ibid.
51 Timothy Goldsmith, “Optimization, Constraint, and History in the Evolution of Eyes,” The Quarterly Review of Biology 65:3 (Sept. 1990): 281–2.
52 Ibid., 287
53 Kang Zhang, E. Nguyen, A. Crandall, and L. Donoso, “Genetic and Molecular Studies of Macular Dystrophies: Recent Developments,” Survey of Ophthalmology 40:1 (1995): 51–61.
54 Richard Young, “Sunlight and Age-Related Eye Disease,” Journal of the National Medical Association 84:4 (1992): 354.
55 Ehud Zamir, “Central Serous Retinopathy Associated with Advenocorticotrophic Hormone Therapy,” Graefes Archives for Clinical Ophthalmology 235 (1997): 339–44.
56 Hewitt and Adler, “The Retinal Pigment Epithelium and Interphotoreceptor Matrix: Structure and Specialized Functions,” 67.
57 Ariel Roth, Origins (Hagerstown, MD: Review and Herald, 1998), 108–9.
58 M. Ali and A. Klyne, Vision in Vertebrates (New York: Plenum Press, 1985).
59 C. Leon Harris, Concepts in Zoology (New York: Harper Collins, 1992).
60 Ali and Klyne, Vision in Vertebrates, 125.
61 Lael Wertenbaker, The Eye: Window to the World (New York: Torstar Books, 1984).
62 Prince, Comparative Anatomy of the Eye.
63 Sophie M. Raymond and Ian J. Jackson, “The Retinal Pigment Epithelium is Required for Development and Maintenance of the Mouse Neural Retina,” Current Biology 5 (1995): 1286.
64 Alfred Coulombre, “Roles of the Retinal Pigment Epithelium in the Development of Ocular Tissue,” chap. 4 in The Retinal Pigment Epithelium.
65 Prince, Comparative Anatomy of the Eye, 343.
66 Roth, Origins, 109.
67 D. A. Baylor, T. D. Lamb, and K. W. Yau, “Response of Retinal Rods to Single Photons,” Journal of Physiology 288 (1979): 613–34.
68 Robert Ferl and Robert A. Wallace, Biology: The Realm of Life (New York: Harper Collins, 1996), 611.
69 Fritiof Sjostrand, “An Elementary Information Processing Component in the Circuitry of the Retina Generating the On-Response,” Journal of Ultrastructure and Molecular Structure Research 102 (1989): 24–38.
70 Young, “Sunlight and Age-Related Eye Disease,” 355–7.
71 Tortora and Grabowski, Principles of Anatomy and Physiology, 461.
72 Young, “Sunlight and Age-Related Eye Disease.”
73 Richard Lumsden, “Not So Blind a Watchmaker,” CRSQ 31 (1994):13–21.
74 Williams, Natural Selection: Domains, Levels, and Challenges, 73.
75 Richard Dawkins, Climbing Mount Improbable (New York: W. W. Norton, 1996), 170.
76 Hewitt and Adler, “The Retinal Pigment Epithelium and Interphotoreceptor Matrix: Structure and Specialized Functions,” 67.
77 Frank Gill, Ornithology (New York: W. H. Freeman, 1995), 189.
78 Ibid., 190.
79 Prince, Comparative Anatomy of the Eye.
80 Jonathan Sarfati, “A review of Climbing Mount Improbable by Richard Dawkins,” Cen Tech J. 12:1 (1998): 33 and Carl Wieland, “Seeing Back to Front,” Creation 18:2 (March– April 1996): 38-40.
81 Hamilton, “The Retina of the Eye—An Evolutionary Road Block,” 63.
* ASA Fellow