 Science
in Christian Perspective
Science
in Christian Perspective Science
in Christian Perspective
Science
in Christian Perspective
Do
Phyletic Lineages Evolve from the Bottom Up
or Develop from the Top Down?
Robert F. DeHaan*
7714 McCallum Street
Philadelphia, PA 19118-4308
From: Perspectives on Science and Christian Faith 50 (December 1998): 260-271.
There are two logical possibilities of how species and large groupings of animals, called phyla, changed over geologic time. The first is from the bottom up, i.e., from varieties, species, genera, families, up to higher taxa. The second is from the top down, from higher taxaˇphyla, classes, ordersˇdown to species. This article reviews and evaluates current data and arguments purporting to confirm the bottom-up framework. The top-down concept is examined considering the data on phyletic trends following the Cambrian explosion as detected in the fossil record. Paleontological and biological data substantiate the top-down direction of change. A new theoretical framework, based on principles of development, is proposed to account for the top-down trends.
This paper will evaluate two fundamental hypotheses regarding how phyletic lineages changed over geologic time. A phyletic lineage is a genetically continuous animal group existing over long periods of time. The first hypothesis may be called the "bottom-up" hypothesis. Proposed by Darwin, it claims that lineages start with varieties and species which become modified "upward" into higher taxonomic levels.1 The second hypothesis is designated the "top-down" hypothesis in which phyletic lineages, starting at the top of the taxonomic hierarchy, differentiate "downward" to the lower taxonomic levels.2 The first represents the Darwinian paradigm; the second embodies the hierarchical developmental perspective.
Both hypotheses cannot be true. Since sufficient physical data are now available, a definitive judgment can be made about which one most closely matches the biological and paleontological data. The confirmation of one will likely disconfirm the other.
Hierarchies in Organic Life
The terms "top-down" and "bottom-up" draw their meaning from the natural hierarchies in organic life. Hierarchical organization is a fundamental characteristic of organic life,3 although such organization is not always easy to detect because the data disclosing it tend to be "messy" and are almost swamped by "noise." Arthur has identified three natural hierarchiesˇmorphological, genealogical, and genetic.4 This article will use the first two. A morphological hierarchy is a linear or nearly linear ranking of animals and plants in terms of form and structure, with organisms at each level possessing morphological characteristics of those above it, but none of the features of those below. The first putative chordates, for instance, did not have any appendagesˇfins were added later, later legs, still later fur. A genealogical hierarchy is a regular chronological descent of a group of organisms from a progenitor or ancestor down to the last members of the group, usually diversifying along the way.
Using both morphological and genealogical hierarchies in a mixed fashion, Linnaeus constructed a system for classifying plants and animals which is known today as the Linnaean hierarchy. Although greatly modified through the years, it is the internationally accepted system of taxonomic nomenclatureˇwith the taxonomic category phylum at the top of the animal kingdom, followed by class, then order, family, genus, and species. (See Appendix A, p. 269.) The Linnaean hierarchy will be used in this article with the understanding that it is based on the natural morphological and genealogical hierarchies.
In the Linnaean system, the topmost organisms in the natural or morphological hierarchy of a group of animals are accorded the rank of phylum.5 The most distinguishing feature of the phylum is the body plan or basic architecture. Exhibited in Cambrian animals, which appeared around 525 million years ago, the body plan is passed on to all their progeny and is the identifying characteristic of the phylum or lineage. Worms, insects, and mammals, for instance, have basic body plans characteristic of their phylum. The top-down direction thus starts at the phylum level. At each lower level in the taxonomy, more specific morphological features are added to the lineage. At the bottom of the natural hierarchy lie the most specific organisms which, in the Linnaean system, are called species or varieties. The bottom-up direction works in the opposite direction. According to Darwin, it starts with species, in the Linnaean sense, and works upward to higher taxa, orders, classes, and phyla.
The Bottom-Up DirectionˇThe Darwinian Perspective
Darwin's Diagram
Darwin's diagram marks
the birthplace of the "bottom-up" concept of evolution, also called
the "specific-to-general" direction of phyletic
change.6 It provides the clearest picture of the
purported evolutionary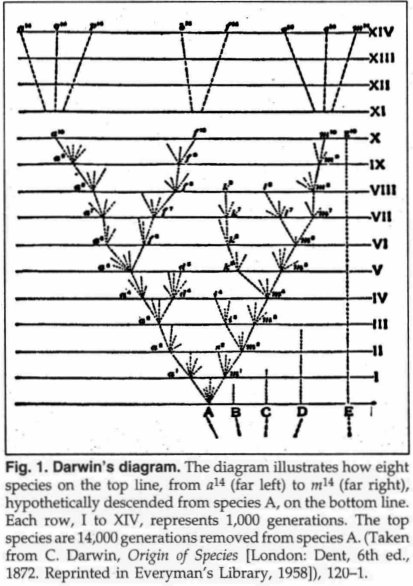 direction of lineal modification, with the branching
pattern of speciation as depicted in Fig. 1.
direction of lineal modification, with the branching
pattern of speciation as depicted in Fig. 1.
Darwin explained his diagram as follows:
Thus the diagram illustrates the steps by which small differences distinguishing varieties are increased into larger differences distinguishing species. By continuing the process for a greater number of generations...we get eight species marked by the letters between a14 and m14 all descended from (A). Thus, as I believe, species are multiplied and genera are formed (My emphasis).7
Darwin next extended the process of modification of species to the taxonomic levels of families and orders, by means of natural selection. He states:
I see no reason to limit the process of modification, as now explained, to the formation of genera alone...These two groups of genera will thus form two distinct families, or orders, according to the amount of divergent modification supposed to be represented in the diagram (My emphasis).8
These clear, unambiguous statements constitute Darwin's hypothesis of how major evolutionary change should occur. What is predicted by his hypothesis is spelled out in the following six elements:
1. Evolution starts at the bottom of the natural hierarchy, with varieties and Linnaean species at the very lowest taxonomic level ("small differences distinguishing varieties").9
2. Numerous species will be formed ("species are multiplied").
3. The process is a long one (implied by many generations of species).
4. Species will diverge from each other ("divergent modification").
5. New higher taxonomic innovations will result ("genera will be formed...two groups of genera will thus form two distinct families or orders").
6. The hypothetical process proposed by Darwin is speciation (the formation of species); the mechanism is natural selection (random genetic variation, resulting in animals which are sorted out by the environment) so that the most adaptive, reproductively successful individuals and populations survive.
It needs to be emphasized that it is the direction of phyletic change that is being evaluated in this paper. Secondarily, Darwin's process of speciation and the mechanism of natural selection are also evaluated. Natural selection is the linchpin of evolutionary theory, the major Darwinian mechanism of change in the organic world.
While Darwin's interpretation of his diagram began as a hypothesis, today it is treated by many as a fact and has become an essential element in the established Darwinian paradigm. Below are some statements illustrating the widespread acceptance of Darwin's doctrine as a statement of fact.
After a lengthy and convoluted discussion of speciation, Simpson, a major author of the Modern Synthesis of evolution, concluded:
Phylogenetic splitting of lineages, including those from which higher categories up to the highest later develop, thus occurs by speciation at their bases (My emphasis).10
Mayr, another architect of the Modern Synthesis and a major evolutionary author, stated:
The origin of new species...is the most important single event in evolution.
The species are the real units of evolution...And speciation, the production of new gene complexes capable of ecological shifts, is the method by which evolution advances. Without speciation there would be no diversification of the organic world, no adaptive radiation, and very little evolutionary progress. The species, then, is the keystone of evolution.11
The Darwinian notion of the branching, bottom-up direction of change, leading to higher animal groups, is reaffirmed by Eldredge:
In his only diagram in On the Origin of Species, Darwin depicted the results of his process of "descent with modification" as a historical, branching pattern. New branches arise from old...A hierarchical array of evolutionary noveltiesˇhomologiesˇautomatically results from the simple process of branching and descent with modification. This pattern, in fact, is the most important prediction about the way the biological world is structured that arises from the scientific hypothesis of "evolution."12
This statement needs clarification. The phrase, "hierarchical array of evolutionary novelties" means new, higher taxa; and "automatically results from the simple process of branching and descent with modification" means forming species by means of natural selection. Eldredge claims that Darwin's diagram is his most important, if not indispensable, prediction. The centrality of the bottom-up hypothesis is clearly established by Darwin and his followers.
Some non-Darwinian authors have also accepted the bottom-up track of evolution. Martin asserted:
Evolution begins with the production of new species, which gradually differ more and more from each other until new genera, families, classes, etc., have evolved.13
Edwards stated:
A population may undergo continuous evolutionary change that can result in the origin of new varieties, species, genera, or indeed new populations at any taxonomic level.14
In summary, Darwin's hypothesis that evolution works from the bottom up is clearly formulated and has become more than a hypothesis. It could be called the central dogma of Darwinism. It is held almost universally in the scientific community and among large segments of the general population.
Do Data from Biological Studies Confirm the Bottom-Up Direction?
In a recent article in Natural History, Gould reviewed several studies that purport to reveal how evolution works.15 Briefly, the first study by Resnick showed that guppies in Trinidad which inhabited high-predation pools bred faster and more copiously, matured faster, and were smaller than guppies living in low-predation pools.16 The explanation is that the threat of predation favors rapid sexual maturation. Thus guppies reproduce in large numbers before being eaten. When the experimenter transferred guppies from high-predation downstream pools into low-predation upstream waters, they quickly (4˝11 years) adopted a more relaxed lifestyle because the threat of predation was gone. This new lifestyle resulted in delayed sexual maturity, growth to a larger size, and a longer life.
A second study by Losos showed how lizards, living on a Bahamian island covered with large trees and thick branches which served as perching places, had evolved long legs. When these lizards were transferred to another island with bushy, narrow, twiggy growth, in less than twenty years, they evolved slightly shorter legs which were more adapted to these precarious perches.17
Other short-term evolution studies have been reported, such as Kettlewell's famous studies of industrial melanism in peppered moths;18 Schluter's studies of a population of sickleback fish which began to change shape and feeding habits when a new competitor forced the fish toward a different ecological niche;19 Endler's observations of guppies in the wild, which when living in streams with predatory fish, blended with the sand on the stream bed for camouflage, whereas in streams lacking predators guppies displayed more visible colors, with spots bigger than sand grains;20 and Vrijenhoek's finding that sexually and asexually reproducing lines of topminnows vary in their resistance to flatworms which burrow into their bodies and give them black spot disease.21
Weiner extrapolated these various studies of short-term evolution into a model of how the history of life worked, i.e., how microevolution operated from the bottom up to produce major innovations in vertebrates, fish, amphibians, insects, and human beings. Referring to these short-term evolution studies, he wrote: "The history of these radiations is the history of life."22 His interpretation is probably widely accepted in the scientific community.
Do these studies provide a model for bottom-up evolution? Gould thinks not. He concluded that in all of these studies, microevolution occurs at rates far too rapid to serve as models for bottom-up evolution. He wrote:
These shortest-term studies are elegant and important, but they cannot represent the general mode for building patterns of life history...Evolutionary rates of the moment as measured by guppies and lizards, are vastly too rapid to represent the general modes of change that build life's history through geological ages...These measured changes over years and decades are too fast by several orders of magnitude to build the history of life by simple cumulation.23
Resnick stated:
The estimated rates [for guppies] are...four to seven orders of magnitude greater than those observed in the fossil record "(that is, ten thousand to ten million times faster!)."24
Even more important is the fact that these studies show that the changes are minor, oscillating, and transient. As Gould concluded:
Most cases such as the Trinidadian guppies and Bahamian lizards represent the transient and momentary blips and fillips that "flesh out" the rich history of lineages in stasis (My emphasis).25
Niles Eldredge, curator of the American Museum of Natural History, challenged the misinterpretation and unwarranted extrapolation of microevolution into macroevolution as follows:
It is dawning on us all, geneticists and paleontologists alike, that the constant genetic churning within individuals, and even within populations, does not mean that the constantly running motor of genetic change will necessarily alter the way a species looks even through long segments of geologic time. Rather than assuming that the small-scale changes necessarily add up, inevitably, to large-scale change as the geologic ages roll, many of us now see that evolution is a hierarchical processˇand that what happens at one level need not specify what goes on at the next higher level.26
Darwin's Finches
Thirteen species of
Darwin's finches live on the GalĚpagos Islands.27
They arrived earlier than other birds and encountered an abundance of unoccupied
ecological niches. The finches thus would have undergone extensive adaptive
radiation, evolving a variety of species and probable genera, which could
exploit opportunities for living such as are exploited by other kinds of birds
in balanced continental fauna.28 The size and
conformation of the beak are adaptively adjusted to the kind of food on which a
given bird depends. Fig. 2 illustrates the diversity of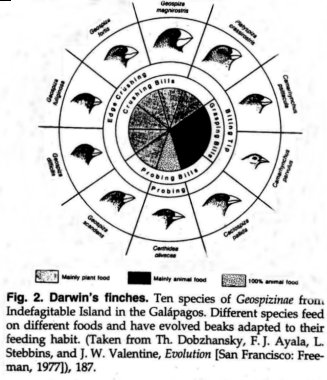 feeding habits of these
species of finches, and also their variety of beak shapes.
feeding habits of these
species of finches, and also their variety of beak shapes.
No higher taxa, however, have been formed, only additional species and genera. To extrapolate these minor microevolutionary modifications at the species level into major innovations at the level of higher taxa is unjustified.
In conclusion, Darwin's hypothesis finds slight empirical support in his finches' study at the lower taxonomic levels (species, genera), but none at the higher taxonomic levels (orders, classes, phylum). His hypothesis finds no support in short-term evolution studies. First, changes depicted in these studies are minor, transient, and occur far too rapidly to be considered the first step in the bottom-up direction. Second, no higher taxa at the level of orders and above have emerged in these studies.29 They fail to substantiate the indispensable element in Darwin's hypothesisˇthe production of higher taxa by means of speciation and natural selection. Williams concluded: "Speciation in the usual sense (of Mayr) has no special significance for macroevolution."30 The bottom line of this review is that Darwin's hypothesis fails to be supported at the higher taxonomic levels.
The Top-Down View
The Cambrian Explosion
The Cambrian explosion, called by some "The Big Bang of Animal Evolution," provides an excellent introduction to the discussion of the top-down hypothesis.31 Life took an enormous leap forward in this explosion, starting 530 million years ago and ending five million years later32ˇa mere eye-blink in the totality of the three billion-plus years that life existed on earth. An amazing number of fifty Cambrian animals sprang upon Earth's stage in that small window of time. Moreover, each animal was unique and distinct from all others. These founding animals are accorded the top-level rank, phyla and classes, within the animal kingdom in the Linnaean system. The Cambrian animals were the ancient founding parents of all but one (Bryozoa) of the major groupings of animals, 37 of which have survived to the present time.33 This then is where the top-down direction of change had its origin.
Each of the fifty animals possessed its own unique structural architecture or body plan, which became the identifying characteristic of the phylum they founded. They bequeathed this body plan on all their progeny. Even today it shows up in the embryonic stage of every one of their offspring and is the distinguishing mark of each phylum.34
In sum, four characteristics mark the Cambrian explosion from all other events in the history of life: (1) its amazing speed (five million years), (2) its incredible breadth (fifty disparate, unique animals), (3) its finality (only one additional phylum formed after this time), and (4) its significance (it is the point of origin of virtually all animal phyla).
The Neoproterozoic
The period prior to the Cambrian explosion is called the Precambrian or Neoproterozoic. For a long time, we knew practically nothing about it. Recent discoveries have produced four extraordinary findings: (1) exquisitely preserved, tiny fossil embryos were found in China;35 (2) microscopic sponges, among the earliest of living multicellular animals, also were found in China;36 (3) the latest molecular clock study indicates that a key branch-point in the tree of life occurred about 670 million years ago,37 much later than a previous study had concluded;38 and (4) groups of animals of possible affinity to the Cambrian animals were found in the Neoproterozoic.39 Space limitations prevent a discussion of these remarkable findings.
The discoveries of these Neoproterozoic precursors do not reduce the importance of the Cambrian explosion.
40 Gould called the Cambrian explosion a "rapid spurt of anatomical innovation within the animal kingdom,"41 perhaps using various "developmental patterns"ˇas Vermeij called themˇfound in Neoproterozoic life.42 Arthur concluded that "the explosion around the base of the Cambrian produced the body plans on which all of today's phyla are based...Even if it turns out that the main lineage divergences occurred much earlier."43 As Vermeij claimed: "The new work in no way diminishes the significance of the Vendian-Cambrian revolution."44 Moreover, to my knowledge, no one has provided an explicit explanation of how Darwin's hypothesis could account for the new Neoproterozoic findings.More important, the existence of embryos, tiny sponges, and other animals in the Neoproterozoic tell us that the process of individual development was already well-established and fully operational.45 This evidence strongly suggests that development did not evolve by Darwinian processes and mechanisms, as some developmental evolutionists maintain.46 Rather, it was already a robust, independent process at the start-up of complex, multicellular life and was at least one of the critical causal factors in the origin of that life.47
Top DownˇThe Developmental Perspective
The top-down direction of change refers to the course of events following the Cambrian explosion, in which thirty-seven of the original fifty phyletic lineages advanced on their long geological journey, from the early, top-hierarchical levels to the later, lower ones. Each phylum was built in the shape of a step-pyramid in Egypt. The capstone of each phylum consisted of the Cambrian stem animal whose body plan identified the phylum or lineage it engendered. Organisms at each lower level possessed morphological characteristics of those above it, but none of the features of those below. Each step down added more morphological features of class, order, etc.; larger numbers; and more diversity in the lineage. The process of differentiation proceeded thus until the bottom of the hierarchy was reached, represented by numerous Linnaean species and varieties.
A study of the origin and early differentiation of phyletic lineages of a large data base of skeletonized, invertebrate, marine fossils which existed during the 250 million year era following the Cambrian explosion, called the Paleozoic, was conducted by Erwin, Valentine, and Sepkowski.48 They found that all fossils could be classified into eleven distinct phyla using the Linnaean classification system. Seven of the eleven phyla appeared within the first twenty-five million years of the Paleozoic era, indicating their origin at or near the Cambrian explosion.
The eleven phyla could next be subdivided into 62 classes, which is the next lower category in the Linnaean system. Class-level animals (not including some stragglers) emerged over a period of 230 million years, with the midpoint at about eighty million years after the start of the Paleozoic, and fifty-five million years later than the midpoint of the distribution of phyla.
The
top-down direction of change refers to the course of events
following the
Cambrian explosion, in which thirty-seven of the
original fifty phyletic
lineages advanced on their long geological journey,
from the early,
top-hierarchical levels to the later, lower ones.
The classes, in turn, could be subdivided into 307 orders (the next lower, more specific taxonomic category) with many more members than the higher categories. The midpoint of the distribution was at 190 million years after the start of the Paleozoic, which is 110 million years later than the midpoint of classes, and 165 million years after the midpoint of the phyla. The authors concluded:
Most higher taxa were built from the top down, rather than from the bottom up. The fossil record suggests that the major pulse of diversification of phyla occurs before that of classes, classes before that of orders, orders before that of families...the higher taxa do not seem to have diverged through an accumulation of lower taxa (My emphasis).49
Each hierarchical level, therefore, differentiated out of the one preceding it: Classes are derived from phyla; and orders emerge from classes. Finally, the number of lower-level taxa increased in each lower distribution. Each phylum differentiated, on the average, into 5.6 classes; each class, on the average, into 4.9 orders. This threefold process has the shape of the step-pyramid; (1) top-down differentiation, (2) emergence of lower out of higher groups, and (3) spreading out and diversification at each lower level. These data are what the top-down, developmental framework would predict, but not what Darwin's hypothesis anticipated.
The data on marine invertebrates presented above were reworked and extended down to the species level by Signor.50 His study shows that the major pulse of species formation came after the higher taxonomic levels were all in place. Species diversity thus lags behind rather than leads the way. According to Signor, species richness increased by a factor of five in the Cenozoic (the most recent geological era which includes the present). It is estimated that 605,000 fossilized marine invertebrate species existed during the Cenozoic (65 million years in length) versus 44,000 species during the Cambrian period about 530 million years ago (an almost equal period of 60 million years), or almost 14 times more species in the Cenozoic. Signor wrote that there have been as many species in the Cenozoic as there were in all the previous 500 million years. As Valentine said:
A striking aspect of the Lower Cambrian faunas is that although diversity of higher taxa was great, species diversity was low. This appears not to be an artifact of the record...Reconstructions of diversity patterns provide estimates of standing species diversities of only a few thousand during earlier Cambrian stages.51
Species multiplied after, not before, all the higher taxa were in place. This is a significant confirmation of the top-down hypothesis and a cogent challenge to Darwin's hypothesis. This trend of delayed increase in species abundance helps explain why short-term evolution studies produce only species but no higher taxa. Higher taxa are already in place, produced by top-down phyletic-developmental processes. All that remains for natural selection to produce is more species diversity.
Signor's
study shows that the major pulse of species formation
came after the higher
taxonomic levels were all in place...
This is a significant
confirmation of the top-down hypothesis
and a cogent challenge to Darwin's
hypothesis.
Other research on the fossil record supports the top-down, general-to-specific direction of change. These studies are important because they show that the top-down pattern of phyletic development and growth occurred (1) in other animal groups besides the well-skeletonized marine invertebrates, (2) at lower taxonomic levels of families, genera, and species, as well as in higher ones of phyla, classes, and orders; and (3) in later geological periods as well in the Cambrian.
Among trilobites, an extinct group of arthropods, the top-down pattern of change occurred at the taxonomic levels of families and genera. Valentine reported the top-down direction as follows: Families of trilobites peaked early in the Cambrian and genera crested late in the Cambrian. The proportion of families to genera is highest in the early Cambrian. Later the proportion reverses itself and genera outnumber families; there are roughly 7.5 times as many genera as families. He concluded: "Little or no real evolutionary advance was taking place."52
The top-down pattern of change is also found in amphibia, reptiles, and mammals at the taxonomic levels of orders, families, and genera. Over the latter half of the Phanerozoic, Simpson found that orders peaked before families, and families before genera among amphibians and mammals. In discussing Simpson's results, Padian and Clemens stated:
Classes appeared in the fossil record some 25 to 30 million years before they achieved maximum ordinal diversity; after a similar interval, the orders achieved maximum generic diversity (My emphasis).53
Not only did amphibians and mammals develop from the top down, they also proliferated as they differentiated. There are roughly five times as many families as orders, and about ten times as many genera as families. The sequence of appearance is less clear among reptiles.
Simpson also suggested the general-to-specific direction of phyletic change. Regarding the general order of appearance of different taxonomic levels, he observed:
If time frequency curves are plotted for the same group in terms of different taxonomic levels, the peaks for higher categories usually appear earlier than those for lower categories..Even when using the coarse scale of periods, peaks for different categories are in the same period, those of higher categories are earlier in the period as the data from mammals show.54
Birds developed very rapidly and also followed the general-to-specific direction of differentiation. Feduccia observed:
This explosive evolution paralleled that of mammals, producing all the modern lineages of birds within about 10 million years, yielding modern orders by the Paleocene and Eocene, modern families by the late Eocene or early Oligocene, and modern genera by the Miocene (My emphasis).55
The general-to-specific pattern of change is thus replicated among complex vertebrates at lower taxonomic levels throughout much of the Phanerozoic as well as among invertebrates at higher taxonomic levels. Roger Lewin, a well-known science writer, wrote:
Several possible patterns exist for the establishment of higher taxa, the two most obvious of which are the bottom-up and the top-down approaches. In the first, evolutionary novelties emerge, bit by bit...The Cambridge explosion appears to conform to the second pattern, the top-down effect.56
In 1952, Goldschmidt summarized the top-down view when he described the direction of change in the fossil record after the Cambrian explosion as follows:
A phylum consists of a number of classes all of which are basically recognizable as belonging to the phylum but, in addition, are different from each other. The same principle is repeated at each taxonomic level. All genera of a family have in common the traits which characterize the family...So it goes down to the level of species. Can this mean anything but that the type of phylum was evolved first and later separated into the types of the classes, then into orders, and so on down the line?57
In summary, phyletic groups, originating in the Cambrian animals, developed in geologic time in the top-down direction. Phyla differentiated downward to each lower level, and increased in number and diversity of representative organisms at each level. Higher taxonomic levels were in place before species multiplied in large numbers. Major innovations preceded minor variations, contrary to Darwinian predictions. Evidence from the fossil record supports the conclusion that the pervasive direction of change and modification works from the top-most, general levels to lower, more specific ones. Based on empirical studies, the conclusion supports the top-down hypothesis, and moreover, contradicts the direction of change predicted by Darwin's diagram.
Objections to the Top-Down Interpretation
Simpson, a prominent evolutionary author, has not hesitated to mount vigorous attacks on the top-down interpretation of paleontological data. First, he dismissed the straight-forward interpretation that peaks of higher taxa appeared in the fossil record before peaks of lower ones as, "off-hand...a manner of speaking, a broad and figurative view of the net result rather than a description of the process, even an artifact of classification."58 To imply, however, that the differentiation of marine invertebrates, described by Erwin, et al., is an "artifact of classification," rings hollow when the authors, who are committed evolutionists, consciously used taxonomic classifications, stating:
The fossil record suggests that the major pulse of diversification of phyla occurs before that of classes, classes before that of orders, orders before that of families.59
Second, Simpson asserted that since every higher taxonomic category is also a species, then by definition, higher taxa originated as species. Referring to adaptive radiation of the family of finches (Geospizidae) on the GalĚpagos, he stated: "The family did not arise as such, but as a species."60 This, however, is not the way Darwin used the concept of species, nor is it what he meant. Darwin postulated a long-branching series of numerous Linnaean speciesˇnot just a single nominal speciesˇchanging over time, finally becoming higher taxa.
Mayr
rejected the biological reality of higher taxonomic categories,
stating that
they are largely arbitrary and artifactual.
Third, Mayr rejected the biological reality of higher taxonomic categories, stating that they are largely arbitrary and artifactual. He attacked the position of Goldschmidt presented above by stating:
With this interpretation Goldschmidt has fallen into the error of considering these categories something natural rather than (particularly in the crucial area of branching) a man-made artifact.61
Of course! Categories of thought, even language itself, are human artifacts. Behind the Linnaean system, however, lie the natural morphological and genealogical hierarchies which they represent, albeit imperfectly. Mayr's criticism should also apply equally to Darwin, who freely used the taxonomic categories of species, genera, families, and even orders in formulating his bottom-up hypothesis.
Parallel Between Phylogenetic and Developmental Hierarchies
Phyletic lineages are hierarchically organized; animals at the highest levels of the morphological hierarchies of phyletic lineages appeared first in the Cambrian explosion, and lower levels differentiated out of higher ones over geologic time. Phyletic hierarchies are evident in the fossil record and are reflected in the Linnaean classification system.
Are morphological hierarchies also evident in the embryonic development of individual animals (ontogeny)? If so, do they correspond in any way to the hierarchies in phylogeny? Answers to these two questions are difficult to determine from the Neoproterozoic, where most of the phyla probably had their roots. Determining the origin of phyla before the Cambrian explosion and their genealogical relationships to each other is problematic because of the difficulties and disagreements in interpreting microbiological findings and meager fossil data.62 Despite these difficulties, Arthur saw a parallel between early phylogeny and early embryology. He states:
Both ontogeny and phyologeny are, in certain respects, hierarchical processes...There is a period of intense morphogenic creativity [in individual embryology, DH] associated with the early branching of the cell lineage hierarchy extending from cleavage and gastrulation up to early organogenesis. This is a broad parallel with early body-plan creativity associated with early genealogical branching of phyletic lineages...The taxonomically broadest characters are also the embryologically earliest.63
Valentine and Erwin stated the same thing more simply: "There is a fairly clear general parallel between developmental patterns and the patterns of distinctiveness of adult body plans of [Cambrian] animals."64 A perfect parallel, however, is not to be expected since many random events affect phyletic patterns over geologic time.
The
characteristics of the fossil embryos are similar
to the characteristics of
modern embryos.
This suggests that development was at least a major causal
factor
in the origin and early development of phyla.
The relationship between phyeltic and individual embryonic development was proposed long before Darwin's day. In 1844 Chambers stated:
Here we have very clear demonstrations of a parity, or rather a identity, of laws presiding over the development of animal tribes [phyletic groups, DH] on the face of the earth, and that of the individual in embryo.65
We have noted above that developmental processes were already well established in the Neoproterozoic as evidenced by the earliest fossil embryos found in China. The characteristics of the fossil embryos are similar to the characteristics of modern embryos. This suggests that development was at least a major causal factor in the origin and early development of phyla.
In terms of the body plan, the parallel between individual embryological development and phyletic development is spelled out in greater detail below:
1. Primacy of the body plan. The body plans of individual animals are among the very first anatomical structures to appear in the embryo. The body plans of Cambrian animals were established early in the history of phyletic lineages. They were among the very first phyletic structures to appear in the Cambrian explosion.
2. Rapid origin of body plans. In individual human development, the basic body plan, including the major organ systems, develop rapidly within the first couple of months after fertilization of the egg. The Cambrian animals with their body plans emerged within a period of five to ten million years, which amounts to something less than one percent of the 525 million years or so that have elapsed since.
3. Stability of body plans. In individual animals, the basic body plans are extremely stable; mutations are lethal or severely detrimental during this early embryonic stage. Minor variations generally appear in later stages of development.66 Body plans of Cambrian stem animals are also extremely stable.67 They have remained essentially unchanged for more than 500 million years, and in the main phyla, across many thousands of species.68 Mutations modify transient characters, e.g., coloration, shape of beaks, but not the basic body plans.
4. Top-down direction of change. The general direction of
embryonic development is from the earliest, most general, most stable features
to later, minor, and specific ones. Phyla also developed hierarchically, from
the top down, as discussed in earlier sections of this paper.
general, most stable features
to later, minor, and specific ones. Phyla also developed hierarchically, from
the top down, as discussed in earlier sections of this paper.
In summary, the primacy of the body plans, their early rise, their rapid formation, and their stability suggest that the early stages of phyletic development result from developmental processes, as in individual development, and are not critically influenced by Darwinian mechanisms. As phyletic development runs its course over geologic time, however, developmental processes gradually give way to natural selection and speciation (microevolution) which add minor adaptive variations to the already established lineage. The relationship of phyletic development to individual development warrants further research.
Philosophical and Theological Implications
Briefly, what are the philosophical and theological implications of this paper?69 Darwinian evolution, as Denton correctly observed, is:
the centrepiece, the crowning achievement, of the naturalistic view of the world, the final triumph of the secular thesis.70
The naturalistic worldview is based on the centrality of Darwinian natural selection.71 This paper challenges the scientific validity of the central Darwinian mechanism of evolution, and, therefore, the centerpiece itself and the naturalistic worldview it supports.
Summary and Conclusions
Darwin's hypothesis, that higher taxa evolved from lower taxa by means of natural selection, finds limited support at the lowest taxonomic levels, but none at the higher levels. Natural selection as shown in microevolution does not appear to have been a major causal factor in the early geologic history of the great groups of animals. Microevolution as exemplified in short-term evolution studies produces only trivial, transient variability, and occurs too rapidly to serve as a model of macroevolution. It is unwarranted for Darwinian authors to extrapolate short-term evolution into macroevolution.
Evidence from paleontology, moreover, indicates that the pervasive pattern of change in the major animal groups is from the top down, not from the bottom up. The top-down hypothesis thus receives strong support from the fossil record. The top-down pattern of individual development parallels the patterns of phyletic development. This suggests that internal developmental processes, not Darwinian mechanisms, constitute the critical causal process accounting for the top-down direction of change in phyletic lineages. How this occurred, however, requires further study.
ę1998
Acknowledgment
Advice on this paper was received from John Wiester and Arthur Battson, for which I am grateful.
References and Notes
1Higher taxa, in this paper, will refer to the taxonomic levels of phyla, classes, orders.
2Lower taxa will refer to taxonomic levels of species, genera, and families.
3Th. Dobzhansky, "Species and Their Origins" in Th. Dobzhansky, F. J. Ayala, L. Stebbins, and J. W. Valentine, Evolution (San Francisco: Freeman, 1977), 168˝9; and W. Arthur, The Origin of Animal Body Plans (Cambridge: Cambridge University Press, 1997).
4W. Arthur, Ibid., 259˝64.
5The term "phylum" is used in two ways that may lead to confusion. First, it refers to the topmost taxonomic category in the Linnaean system; second, it refers to the entire lineage or genealogical grouping of animals.
6C. Darwin, Origin of Species (London: Dent, 6th ed. 1872. Reprinted in Everyman's Library, 1958), 121. This diagram is possibly the most published diagram in the history of evolutionary biology.
7Ibid., 112.
8Ibid., 115.
9Eldredge employs even "lower" levels, starting with germline, organism and demes, the former being composed of hierarchically nested chromosomes, genes, codons, and base pairs. N. Eldredge, Macroevolutionary Dynamics (New York: McGraw-Hill, 1989), 157.
10G. G. Simpson, The Major Features of Evolution (New York: Simon and Schuster, 1953), 383˝4.
11E. Mayr, Animal Species and Evolution (Cambridge, MA: Harvard University Press, 1963), 11, 621.
12Eldredge, Macroevolutionary Dynamics, 1.
13E. A. Martin, The Dictionary of Life Sciences (New York: Pica, 1984), 131.
14P. Edwards, ed., The Encyclopedia of Philosophy, vol. 1 (1967, reprint, New York: Macmillan, 1972), 297˝8.
15S. J. Gould, "The Paradox of the Visibly Irrelevant," Natural History 106 (12/97˝1/98): 12˝8, 60˝6.
16D. N. Reznick, F. H. Shaw, F. H. Rodd, R. G. Shaw, "Evaluation of the Rate of Evolution in Natural Populations of Guppies (Poecilia reticulata)," Science 275 (March 28, 1997): 1934˝7; and V. Morrell, "Predator-Free Guppies Take an Evolutionary Leap Forward," Science 275 (March 28, 1997): 1880 quoted in S. J. Gould, "The Paradox of the Visibly Irrelevant," 15˝6.
17J. B. Losos cited in S. J. Gould, "The Paradox of the Visibly Irrelevant," 16˝8. See also, V. Morell, "Catching Lizards in the Act of Adapting," Science 276 (May 2, 1997): 682˝3.
18H. B. D. Kettlewell, "Selection Experiments on Industrial Melanism in Lepidoptera," Heredity 9 (1955): 341.
19D. Schluter, "Experimental Evidence that Competition Promotes Divergence in Adaptive Radiation," Science 266 (Nov. 4, 1994): 798˝800; and J. Weiner, "Evolution Made Visible," Science 267 (Jan. 6, 1995): 30.
20J. Weiner, "Evolution Made Visible," 30˝3. See also D. N. Reznick, et al., "Evaluation of the Rate of Evolution," 1934˝7.
21J. Weiner, "Evolution Made Visible," 32.
22J. Weiner, The Beak of the Finch (New York: Random House, 1994), 208.
23S. J. Gould, "The Paradox of the Visibly Irrelevant," 62, 64.
24Quoted in V. Morrell, "Predator-Free-Guppies Take an Evolutionary Leap Forward," Science 275 (March 28, 1997): 1880.
25S. J. Gould, "The Paradox of the Visibly Irrelevant," 64.
26N. Eldredge, "What Drives Evolution?" Earth (December 1996): 37.
27P. Grant and B. Rosemary Grant, "Hybridization of Bird Species," Science 256 (April 10, 1992): 193˝7; J. Weiner, The Beak of the Finch, 17; and see also Th. Dobzhansky, "Species and Their Origins," 186˝7.
28P. Grant and B. Rosemary Grant, "Hybridization of Bird Species," Science 256 (April 10, 1992): 193˝7. Peter and Rosemary Grant reproduced and extended Darwin's original studies of finches, and reported that changes in the type of available seeds produce changes in the beak size and shape of the finches within a single year.
29S. F. Gilbert, J. M. Opitz, and R. A. Raff gave this pithy evaluation of microevolution [short-term evolution]: "Microevolution looks at adaptations that concern only the survival of the fittest, not the arrival of the fittest" (my emphasis) in "Resynthesizing Evolutionary and Developmental Biology," Developmental Biology 173 (1996): 361.
30C. H. Williams, Natural Selection, Domains, Levels and Challenges (London: Oxford University Press, 1992) in G. L. G. Miklos, "Emergence of Organizational Complexities During Metazoan Evolution: Perspectives from Molecular Biology, Paleontology and Neo-Darwinism," Mem. Ass. Australas Paleontols 15 (Sept. 9, 1993): 26.
31J. S. Levinton, "The Big Bang of Animal Evolution," Scientific American 267 (November 1992): 84˝91.
32Bowring, et al., estimated that the Cambrian explosion occurred in five to ten million years. See S. A. Bowring, J. P. Grotzinger, C. E. Isachsen, A. H. Knoll, S. M. Pelechaty, P. Kolosov, "Calibrating Rates of Early Cambrian Evolution," Science (September 3, 1993): 1293˝8. Jablonski estimated a somewhat longer period: "During a relatively short period of 20 million years, all 37 major body plans in the animal kingdomˇfrom sponges to vertebratesˇarose" in D. Jablonski, "Developing a New View of Evolution," Science 277 (July 4, 1997): 37.
33The discovery of what is claimed to be a new phylum has been reported by P. Funch, and R. M. Kristensen, "Cycliophora is a New Phylum with Affinities to Entoprocta and Ectoprocta," Nature 378 (Dec. 14, 1995): 710˝4. The significance of this discovery still needs to be determined.
34One of the most significant Cambrian animals, from the perspective of the human race, is a little, gracile creature named Pikaia found in the Burgess Shale and in China. Its unique body plan includes striated muscles and the notochord (which becomes the back bone and spinal cord in later progeny). It is considered to be the founding ancestor of all animals belonging to the phylum, Chordata, which includes all mammals and human beings. Thus the chordate body plan, shared by all members of our phylum, goes clear back to Pikaia, in the Cambrian explosion.
35C. Xiao, Y. Zhang, and A. H. Knoll, "Three-dimensional Preservation of Algae and Animal Embryos in a Neoproterozoic Phosphorite," Nature 391 (February 5, 1998): 553˝8; Editorial comment by S. Bengtson, "Animal Embryos in Deep Time," Nature 391 (February 5, 1998): 529˝30; and S. Bengtson and Y. Zhao, "Fossilized Metazoan Embryos from the Earliest Cambrian," Science 277 (September 12, 1997): 1645˝8.
36C. W. Li, J. -Y. Chen, and T. -E. Hua, "Precambrian Sponges with Cellular Structures," Science 279 (February 6, 1998): 879˝82; and R. Kerr, "Pushing Back the Origins of Animals," Science 279 (February 6, 1998): 803˝4.
37F. J. Ayala, A. Rzhetsky, and F. J. Ayala, "Origin of the Metazoan phyla: Molecular Clocks Confirm Paleontological Estimates," Proceedings of the National Academy of Science USA 95 (January 1998): 606˝10.
38G. A. Wray, J. S. Levinton, and L. Shapiro, "Molecular Evidence for Deep Precambrian Divergences Among Metazoan Phyla," Science 274 (Oct. 25, 1996): 568˝73.
39K. B. Miller, "The Precambrian to Cambrian Fossil Record and Transitional Forms," Perspectives on Science and Christian Faith 49 (December 1997): 264˝7.
40It is, of course, to be expected that the Cambrian animals had precursors. See R.<|>F. DeHaan, "Paradoxes in Darwinian Theory Resolved by a Theory of Macro-Development," Perspectives on Science and Christian Faith 48 (September 1996): 154˝63.
41S. J. Gould, "On Embryos and Ancestors," Natural History 107 (July/August 1998): 64.
42G. J. Vermeij, "Animal Origins," Science 274 (Oct. 25, 1996): 525˝6.
43W. Arthur, The Origin of Animal Body Plans, 226.
44G. J. Vermeij, "Animal Origins," 526.
45Researchers are discovering how unbelievably complex the process of development is. See, for example, C. -H. Yuh, H. Bolouri, and E. H. Davidson, "Genomic Cis-Regulatory Logic: Experimental and Computational Analysis of a Sea Urchin Gene," Science 279 (March 20, 1998): 1896˝1902.
46W. Arthur, The Origin of Animal Body Plans, 73; and R.<|>A. Raff, The Shape of Life (Chicago: The University of Chicago Press, 1996), 33.
47When the Cambrian explosion is interpreted from the evolutionary perspective, it is often downplayed as a major discontinuity and played up as just another relatively minor event in the continuous history of animal life.
48D. H. Erwin, J. W. Valentine, and J. J. Sepkowski, "A Comparative Study Of Diversification Events: The Early Paleozoic Versus The Mesozoic," Evolution 41 (1987): 1177˝86.
49Ibid., 1183. Herein lies the origin of the "top-down" and "bottom-up" metaphors.
50P. W. Signor, III, "Real and Apparent Trends in Species Richness Through Time," in J. W. Valentine, ed., Phanerozoic Diversity Patterns: Profiles in Macroevolution (Princeton: Princeton University Press, 1985), 148.
51J. W. Valentine, "Fossil Record of the Origin of Bauplöne and Its Implications," in D. M. Raup, and D. Jablonski, eds., Patterns and Processes in the History of Life (Berlin: Springer-Verlag, 1986), 212.
52J. W. Valentine, "The Geological Record," in Th. Dobzhansky, "Species and Their Origins," 335.
53G. G. Simpson, "Periodicity in Vertebrate Evolution," Junior Paleontology 26 (1952): 359˝70 quoted in K. Padian and W. A. Clemens, "Terrestrial Vertebrate Diversity: Episodes and Insights," in J. W. Valentine, ed., Phanerozoic Diversity Patterns: Profiles in Macroevolution (Princeton: Princeton University Press, 1985), 45˝6.
54G. G. Simpson, The Major Features of Evolution (New York: Simon and Schuster, 1953), 237.
55A. Feduccia, "Explosive Evolution in Tertiary Birds and Mammals," Science 267 (1995): 638.
56R. Lewin, "A Lopsided Look at Evolution," Science 241 (July 15, 1988): 292.
57R B. Goldschmidt, "Evolution as Viewed by One Geneticist," American Scientist 40 (1952): 91˝2.
58G. G. Simpson, The Major Features of Evolution (New York: Columbia University Press, 1953), 238.
59Erwin, et al., "A Comparative Study Of Diversification Events," 1183.
60G. G. Simpson, The Major Features of Evolution (New York: Columbia University Press, 1953), 238.
61E. Mayr, Animal Species and Evolution, 600˝1.
62Age determinations from studies of molecular clocks and from fossil results continue to disagree. See A. Gibbons, "Gene Put Mammals in Age of Dinosaurs," Science 280 (May 1, 1998): 675˝6; and E. Pennisi, "Genome Data Shake Tree of Life," Science 280 (May 1, 1998): 672˝4.
63W. Arthur, The Origin of Animal Body Plans, 256, 260, 14. As mentioned earlier, Arthur found it necessary to posit six hierarchiesˇthree for ontogeny and the same three for phylogenyˇmorphological, genealogical, and genetic. That is, each can be found in individual embryonic development and in phyletic development. He warned, however, that the relationship between ontogenic and phyletic hierarchies is both complex and messy, rather than a neat, clean correspondence.
64J. W. Valentine and D. H. Erwin, "Interpreting Great Developmental Experiments: The Fossil Record," in R. A. Raff, and E. C. Raff, eds., Development as an Evolutionary Process (New York: Liss, 1987), 71.
65R. Chambers, Vestiges of the Natural History of Creation (1844, reprint, New York: Humanities Press, 1969), 202.
66"Mutants affecting early embryological stages survive only in the laboratory. An organism must survive as best it can with its given Bauplan" in J. Z. Young, The Life of Vertebrates, 3d ed. (Oxford: Clarendon Press, 1981), 14.
67R. A. Raff, The Shape of Life (Chicago: University of Chicago Press, 1996), 32, 434; B. Runnegar, "Evolution of the Earliest Animals," in J.<|>W. Schopf, ed., Major Events in the History of Life (Boston: Jones and Bartlett, 1992), 87; and W. Arthur, The Origin of Animal Body Plans, 48.
68W. Arthur, The Origin of Animal Body Plans, 48.
69N. Pearcy, C. B. Thaxton, The Soul of Science (Wheaton, IL: Crossway Books, 1994), 248. They stated that "science and scholarship are never carried on in a philosophical and religious vacuum."
70M. Denton, Evolution: A Theory in Crisis (Bethesda, MD: Adler & Adler, 1986), 357.
71Two most vociferous exponents of this position are R. Dawkins, The Blind Watchmaker (New York: Norton, 1987) and D. Dennett, Darwin's Dangerous Idea (New York: Simon and Schuster, 1995).