The Second Law of Thermodynamics and
"Chemical Evolution" for
the Origin of Life
by Craig Rusbult, Ph.D.
This page builds on the foundation of Entropy
and Evolution which explains why "most thermo-based
arguments [against a general ‘evolution’ of all types, and specifically against neo-Darwinian biological evolution] are clearly wrong." This page
will examine specific questions that are quoted
from the section on Chemical Evolution:
As
explained above [in Biological Evolution], the "ratchet" mechanism
of neo-Darwinian evolution [with harmful mutations producing no major
change in a population, and neutral mutations or rare beneficial mutations
being preserved by selection] can produce an increase in biocomplexity. But
this evolutionary mechanism would not exist before life began, and neither would
the coordinated biological systems that make unusual things
happen inside our bodies, including the formation of specific biomolecules
that are needed to make the systems, in a "chicken and egg" problem. In
addition, some chemical reactions that seem important for life (to make essential
biomolecules) are energetically unfavorable, like a ball rolling uphill.
The questions we'll examine are in the paragraph above. Below you'll find the principles we'll use, in excerpts from "Entropy
and Evolution" that will be reminders (if you've read that page, which
I recommend) and will make sense if you already understand thermodynamics,
or if you're a
fast
learner. There
are no omissions (denoted by ..... below) in the final two sections — "why
some things don't happen" and "how
our bodies make
unusual
things happen"
— because these concepts in these sections are
essential
for understanding the ideas later in this page when we look at problems
that must be solved in a formation of life by Chemical Evolution. Here are excerpts from Entropy and Evolution:
At the micro-level,
a system's entropy is a property that depends
on the number of ways that energy can be distributed
among the particles in the system. Entropy is a measure of probability,
because if energy can be distributed in more ways in a certain state, that
state is more probable .....[so] the chemicals in a system tend to eventually
end up in the particular
state (their equilibrium
state) that can occur in the greatest number of ways when energy
is distributed among its zillions of molecules.
Basically,
the Second Law of Thermodynamics is just a description of probability,
..... stating that during
any reaction the entropy of the universe will increase. .....
The everyday
analogies used by some young-earth creationists — like "a tidy room becoming messy" due to
increasing entropy — are not used in scientific applications of the
Second Law, because entropy is about energy distributions and associated
probabilities,
not macroscopic disorder, and our psychological intuitions about "entropy
as disorder" are often wrong. .....
The
Second Law is simple in principle, but applying it to real systems can
be difficult due to the challenge of determining probabilities at the
microscopic level. During a reaction, the entropy in a system
can increase in two main ways, ..... and if
we think of a system's total entropy as "constraint-entropy plus temperature-entropy" an
entropy change can be due to constraint-change [when
constraint increases, and thus freedom decreases, entropy decreases] or temperature-change [when
temperature
increases, so there is more energy to distribute and thus more ways it can be
distributed, entropy increases], or both. Sometimes
these factors produce opposite effects, with constraint causing entropy
decreases and
temperature causing entropy increases. When this happens
the temperature-change is usually a larger factor, so there is an increase
in the overall entropy of the universe even though "disorder" seems
to decrease, as you'll see in the examples below [omitted here]. .....
why things happen: A
wide variety of common reactions occur when an attractive force pulls particles
closer together, which constrains
them (producing a small decrease of entropy) but increases their kinetic
energy and temperature (producing
a larger increase of entropy) so total entropy increases
even though "disorder" seems to decrease. ..... The overall result
of
these reactions [during astronomical evolution] — which produced hydrogen
atoms and molecules, stars, heavy-nucleus atoms, and planets in solar systems — was
an "evolution" that produced a change from simplicity to complexity,
and an increase in ordered structures at the microscopic and macroscopic levels.
.....
why reactions occur: At
normal temperatures, most reactions are "driven
forward" by the formation of stronger attractive-force interactions between
particles (which is manifested as a temperature increase [in the system
and/or in its surroundings]), not
by a decrease of constraints. .....
why chemical reactions occur: In
the common experiences of everyday life, most reactions are chemical, not astronomical. These
chemical reactions occur because stronger bonds ("stronger
attractive-force interactions between particles") are formed due
to the reaction.
Why some things
don't happen.
If we think of all possible
reactions, and ask "Why do some reactions occur, but others don't
occur?", we find two reasons: • If a reaction would
violate the Second Law, it is thermodynamically
unfavorable and will not occur. • Sometimes a reaction
that could occur (since it is allowed by the Second Law) does not occur because
its
rate of reaction is extremely slow, so it is kinetically
unfavorable. This possibility is why, earlier, I said "chemicals tend
to eventually end up in their [thermodynamically optimal] equilibrium state" but
not "chemicals will end up..."
For example, gasoline and oxygen
can exist in a car's gas tank for years without reacting, even though their reaction
is thermodynamically favorable and in some situations they will react very vigorously. Why? Because
these chemicals must overcome an obstacle — an activation
energy — before they can react, and usually (at normal temperatures
when there is no spark or flame) the collisions between molecules of gasoline
and oxygen don't supply enough energy to let them overcome this obstacle and
become "activated," so they don't react and are in a temporary metastable state. {activation
energy is explained more thoroughly in the appendix}
As with most things in nature,
the results of activation energies can be either bad or good. We want
some reactions to occur but they don't occur due to activation energies, and
we think this is bad. But gasoline doesn't burn in a car's gas tank,
only in the engine, and we think this is good. Activation energies are
also biologically useful because they provide a kinetic obstacle — so
undesirable reactions are prevented, and (as explained below) desirable reactions
can be controlled — and this allows life.
How our bodies make
unusual things happen.
Inside our bodies, reactions
occur that would not occur outside our bodies, and molecules exist that
would not exist outside our bodies. How and why can this happen?
kinetics: Many reactions
that usually are kinetically unfavorable can occur because some proteins,
which are called enzymes, act as catalysts that
increase a reaction rate and "make
things happen" by providing a way
to lower the activation energy and/or bring chemicals together in a spatial
orientation
that
is "just
right" for reacting. Enzymes operate in the context of control
systems that control which reactions do and don't occur, and when. These
control systems are analogous to a thermostat that turns a furnace on
and off, when we do and don't need heat, but are much more complex and
wonderful.
thermodynamics: Many
reactions that usually are thermodynamically unfavorable can occur
when they are coupled with another reaction. For example, if
a biologically useful reaction that is unfavorable (because it produces
a change of -400 in universe-entropy) is combined with a sufficiently
favorable reaction (that produces a change of +500 in universe-entropy)
the overall coupled reaction is favorable,
since it produces an increase of +100 in universe-entropy. Our
bodies use external fuel — the chemical
potential energy stored
in the foods we eat and the oxygen we breathe — to drive these
coupled reactions.
Living organisms in the earth's
biosystem are maintained in their unusual state (having
high chemical potential energy, with low constraint-entropy and normal
temperature-entropy) by using external energy from the sun, with solar
energy first
being directly consumed by plants, which convert it into chemical
potential energy that can be consumed by animals. In both
plants and animals, coordinated biological systems
produce the mechanisms of life — operating
in control systems, coupled reactions, and in many other ways — that
are necessary to make these unusual
things happen.
Now, using the principles of thermodynamics outlined above,
we'll look at "problems
to solve" in a formation of life by chemical
evolution:
1) an evolutionary mechanism
[mutation-and-selection] would not exist before
life began,
2)
and
neither would the coordinated biological systems that make
unusual things happen,
3) including the formation of
specific biomolecules that are needed to make the systems;
4) some chemical reactions that seem important for life are energetically unfavorable.
As
you read the comments below, think about how each of the four problems involves
a
"chicken and egg"
relationship, if life is necessary to produce what is necessary for life.
1) The origin of life requires a minimal complexity that
may exceed what can be produced by natural process, and until a system can
accurately reproduce itself (until it's alive?) the neo-Darwinian evolutionary
mechanisms cannot help a system (or a population of systems) move toward the
minimal complexity that is required for life.
2) A wide variety of biological processes, including reproduction in #1,
require the coordinated biological systems of
life. If
you've read my "Entropy and Evolution" page, you won't expect me
to be quoting Henry Morris favorably, but here it is, when he claims that "The
Second Law of Thermodynamics could well be stated as follows: In any
ordered system, open or closed, there exists a tendency for that system to
decay to a state of disorder, which tendency can only be suspended or reversed
by an external source of ordering energy directed by an informational program
and transformed through an ingestion-storage-converter mechanism into the
specific work required to build up the complex structure of that system.
(1976)" Despite
the many errors in this statement — his claim about Second Law "disorder" is
vague and overgeneralized, and "ordering energy" is
too specific to describe the wide variety of "thermodynamically unusual
things" that occur
in a living organism, and despite a "tendency to disorder" the
entropy (not just the perceived "disorder") can decrease
within an open system, and many reactions produce "order" due to
the simple operation of attractive forces (as explained in why
things happen)* — he does
describe the essential functions of information and mechanisms
in
the
maintenance
of life. But would it be possible for life to begin through
a "replicating
closed auto-catalytic system... [that has] the
complex web of interactions
built in from the outset"? {more} {* Do
you see why a favorable quoting of Morris must be rare? }
3) A
living organism must have biomolecules
that are biologically useful. For example, if a large number of long-chain
proteins did form (despite the problems in #4) they
would contain a wide variety of amino acid sequences, and most sequences
would not produce useful proteins (although the utility of early proteins might
have been low but not zero, and then it improved with time) so
the formation
of a biologically
useful protein seems highly improbable. This
low probability is equivalent to a low configurational entropy, according
to Charles Thaxton & Walter Bradley (*)
who analyze entropy in terms of thermal
entropy and configurational entropy which
depend on "the number of ways energy and mass,
respectively, may be arranged in a system." {* Thaxton & Bradley
are the authors, along with geologist
Roger Olsen, of The Mystery of Life's Origin. } {more}
4) In
a natural origin of life, many important chemical reactions are energetically
unfavorable, like a ball rolling uphill, in reactions to form
organic molecules (some amino acids and all nucleotides) and then to combine
these into long-chain
biomolecules (proteins and RNA). A living organism can make
these unfavorable reactions occur by coupling uphill-reactions with downhill-reactions,
to make the combination energetically favorable. But before an "origin
of life" these coupling mechanisms would not yet be available. {more}
Converting Energy into Useful Functions
In The Mystery of Life's
Origin, Thaxton & Bradley explain how living organisms use biochemistry
to produce a metastable environment (which is not thermodynamically favorable,
since the system's low constraint-entropy is not combined with a high temperature-entropy)
by converting externally available energy into internally useful
functions, in a way that causes the overall entropy change in the universe
(in system + surroundings) to be thermodynamically favorable, consistent with
the
Second
Law. Here
is my summary:
Localized areas of relatively low
system-entropy can be maintained by a flow of energy. For
example, supplying energy to a refrigerator can lower the temperature inside
it, even
though this
would
never
occur (and it would violate the Second Law) under ordinary circumstances. But
as long as energy continues to flow through the refrigerator, and its "mechanism
that produces cold" is operating properly, the low-entropy cold area
can be maintained in a way that is consistent with the Second Law. Similarly,
energy flow through our bodies can — due to the mechanisms that maintain
life, that convert external energy (in photons or food) into biologically useful
functions — maintain
our bodies in a low-entropy state.
Thaxton and Bradley explain
why a "coupling
mechanism" is necessary:
"Maintenance
of the complex, high-energy condition associated with life is not possible
apart from a continuous source of energy. A source of energy alone
is not sufficient, however, to explain the origin or maintenance of living
systems. The additional crucial factor is a means of converting this
energy into the necessary useful work to build and maintain complex living
systems. ...
"An
automobile with an internal combustion engine, transmission, and drive chain
provides the necessary mechanism for converting the energy in gasoline into
comfortable transportation. Without such an "energy converter," however,
obtaining transportation from gasoline would be impossible. In a similar
way, food would do little for a man whose stomach, intestines, liver, or
pancreas were removed. Without these, he would surely die even though
he continued to eat. Apart from a mechanism to couple the available
energy to the necessary work, high-energy biomass is insufficient to sustain
a living system far from equilibrium. In the case of living systems
such a coupling mechanism channels the energy along specific chemical pathways
to accomplish a very specific type of work." {quoted from
the first of the book's three chapters about thermodynamics, which are available
on the web}
Mechanisms
during Three Evolutions
Thaxton & Bradley
end the passage above, "We therefore conclude
that, given the availability of energy and an appropriate coupling mechanism,
the maintenance of a living
system far from equilibrium presents no thermodynamic problems." But
they recognize the important difference between maintenance and origin: "While
the maintenance of living systems is easily rationalized in terms of thermodynamics,
the origin of such living systems is quite another matter."
Let's look at the role
of "mechanisms" in
three types of evolution:
During biological evolution
after life is established, the basic life-allowing
mechanisms already exist, which allows life to continue with
inheritance-replication that is adequate (but not perfect) through many
generations, and during
these generations the mechanisms can increase in variety and complexity through
a neo-Darwinian "ratcheting process" that includes, but is not limited
to, mutation and selection. But
even though an evolutionary increase in biological complexity is compatible
with the Second Law, scientists should still ask "What
types of complexity can be produced, in what amounts, and how quickly?"
In chemical evolution the
basic mechanisms do not exist (thus life
cannot exist) so the mechanisms must be produced (so life can
exist). For
a variety of reasons, producing life seems much more difficult than maintaining
life and increasing its diversity & complexity.
During astronomical evolution,
most reactions are driven by simple attractive forces, so a
mechanism is not needed.
Typically, young-earth creationists
use the Second Law of Thermodynamics to criticize ALL evolution, even though
arguments against two types (astronomical and biological) don't seem valid.
Defenders of evolution correctly
claim that an external source of energy can allow a decrease of entropy within
an open system during any type of evolution. But appeals
to an inflow of energy don't address the important difference between
chemical evolution and biological evolution, between producing a
coupling mechanism (in the first generation of life) and then maintaining it
(through succeeding generations). Even though most thermo-based arguments
are wrong, the questions in this page are scientifically
interesting and currently unanswered.
APPENDIX
A Solution for Chicken-and-Egg
Problems?
The replicating closed auto-catalytic
system described by Stuart A. Kaufmann has the advantage that the
complex web of interactions
is built in from the outset. In essence this view acknowledges
irreducible complexity, that is, the system has to be sufficiently
complex in order for auto-catalytic behavior to emerge. There
is no stepwise evolution of this emergent property; it suddenly
appears (as with all emergent properties) once the polymer complexity
has achieved the threshold
level. Thus, the system is complex and whole from the start. Indeed,
this is what living systems appear to be. { Loren Haarsma &
Terry Gray, in Complexity,
Self-Organization,
and Design }
Thermodynamics
and The Origin of Life
Scientists have proposed a
two-stage process for a natural origin of life: reactions form organic
molecules which combine to make larger biomolecules (like proteins
and RNA), which then self-organize into a living organism. For
the first stage, a major problem is that many essential reactions
are "uphill" in energy. Walter Bradley illustrates
downhill-reactions and uphill-reactions by analogy with a pool table. Here
is my paraphrased summary:
Imagine a hemispherical valley in
the middle of a horizontal billiard table that has no pockets. We
place 10 pool balls on the table, then gently agitate the table. Eventually,
all balls end up in the valley. Without the valley this clustering
would have a low probability (and low entropy), but with the valley
it has the highest probability (and highest entropy). There is
less "apparent disorder" but entropy (of the universe) has
increased, consistent with the Second Law.
Bradley explains that "the
difficulty in getting polymerization condensation reactions is that
our pool table [doesn't have a valley]... it has a hill. ... One
might conclude that the formation of protein and DNA via polymerization
condensation reactions is well nigh impossible. ... The only
solution to this dilemma is to do some very specific work on the system
to assist these balls up the hill. ... This work must be very
carefully done so as to not jar loose the balls that are already there. Thus,
the energy must be selective in getting the balls up the hill while
at the same time not causing the balls there to be removed from their
positions of metastable equilibrium."
Many reactions occur because, as
illustrated in Sections 3A and 3B, a small unfavorable change in entropy
is overcome by a large favorable change in energy. But with many
reactions that are essential for life, the changes in entropy and energy
are both unfavorable. A living organism can make these unfavorable
reactions occur by coupling uphill-reactions with downhill-reactions,
to make the combination energetically favorable. But in an "origin
of life" scenario the coupling mechanisms would not be available. Thaxton & Bradley
say, "While the maintenance of living systems
is easily rationalized in terms of thermodynamics, the origin of
such living systems is quite another matter." The
question of an energy-harnessing mechanism — which was not needed
for astronomical evolution, was available for biological evolution,
but was needed yet not available for chemical evolution — is
discussed earlier. {more about the
origin of life} Information
and Entropy
One problem for a natural origin
of life is that even if biomolecules did form, despite the unfavorable
reactions described above, an extremely small fraction of these
biomolecules would be useful. Thaxton & Bradley claim
that this low probability is a low "configurational entropy" and
they explain the difference between two types of entropy:
"Consider
the case of the formation of protein or DNA from biomonomers in
a chemical soup. For computational purposes it may be thought
of as requiring two steps: (1) polymerization to form a chain
molecule with an aperiodic but near-random sequence, and (2)
rearrangement to an aperiodic, specified information-bearing sequence. The
entropy change associated with the first step is essentially all thermal
entropy change, as discussed above. The entropy change
of the second step is essentially all configurational
entropy change."
Their claim seems credible,
since the number of microstates (the number of different
distributions) associated with all possible biomolecule-sequences
(after Step
1) is much
larger than the microstates associated with a specific biomolecule-sequence
(after Step 2), so there has been a large decrease in probability
(and thus entropy) during Step 2, and this is the "configurational
entropy change." We can also think about this
entropy change as the "information" that is needed to
specify the specific sequence.
The rest of this appendix is detailed versions
of three sections — why some things don't happen,... — in
the main body.
Thermodynamics
and Kinetics
If we think of all possible reactions, and ask "Why
do some reactions occur, but others don't occur?", we find two reasons:
thermodynamic and kinetic. In thermodynamics,
we ask "is a reaction thermodynamically favorable?" In kinetics,
we ask "if a reaction can occur, when will it occur and how quickly?" So
far in this page, we've looked at only thermodynamics. But there is a hint
of kinetics in Section 1 where I waffle by saying
that chemicals "tend to eventually end up in
their equilibrium state" instead of just saying they "will end
up..."?
Why is it wise to waffle when making thermodynamic
claims? Because the principles of thermodynamics let us predict whether
a reaction can occur, and what the equilibrium state would be if it
does occur, but thermodynamics does not say when it will occur (and whether it
will probably occur in a given amount of time), or how quickly. To
help us answer these questions, we can use our observations of "what does
and doesn't happen" plus the principles of kinetics.
As contrasting examples of "how
quickly," the reactions of oxygen with iron and with gasoline are
both thermodynamically favorable, but the oxidation of iron occurs slowly
(in rusting) while the oxidation of gasoline occurs quickly (in a fire).
An
Obstacle that Prevents Reaction
The question, "How quickly?",
can be answered in two ways because "quickly" has two
meanings. The kinetic answer above, re: slow rusting or fast
burning, is about speed of reaction. Another
kinetic answer is about the timing of reaction,
and a more precise question is, "When will a reaction occur?"
Gasoline can exist in a car's gas tank
for years without reacting, so during this time it is not reacting quickly. But
when we start the car's engine, a spark initiates a reaction which is so
fast that it becomes a small-scale explosion when the gases produced by
the reaction are confined within a cylinder of the engine. Why can
the reactive chemicals (gasoline and oxygen) exist for years without reacting? Because
the chemicals must overcome an obstacle — an "activation energy" — before
the fast reaction can occur.
What is activation energy? To
illustrate by analogy, imagine a red ball that is trapped in a transparent
bowl on top of a green hill. 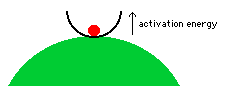 The
ball is thermodynamically unstable because
it would roll down the hill if it could. But this thermodynamically
favorable reaction will occur only if the ball can first escape from the
bowl. The action of the ball climbing up and over the bowl's edge
is analogous to chemicals overcoming their activation energy. Until
this occurs, the ball is in a metastable state;
it is temporarily stable, even though it would react (by rolling down the
hill) if it could. Similarly, the gasoline and oxygen would react,
but they don't react until the spark allows some of the molecules to overcome
their activation energy; this lets them react and they "release
energy" to their neighbors, which lets these neighbors overcome their
activation energy so they can react and release energy to their neighbors,
and so on, in a self-sustaining chain reaction that occurs very quickly,
after it begins. The
ball is thermodynamically unstable because
it would roll down the hill if it could. But this thermodynamically
favorable reaction will occur only if the ball can first escape from the
bowl. The action of the ball climbing up and over the bowl's edge
is analogous to chemicals overcoming their activation energy. Until
this occurs, the ball is in a metastable state;
it is temporarily stable, even though it would react (by rolling down the
hill) if it could. Similarly, the gasoline and oxygen would react,
but they don't react until the spark allows some of the molecules to overcome
their activation energy; this lets them react and they "release
energy" to their neighbors, which lets these neighbors overcome their
activation energy so they can react and release energy to their neighbors,
and so on, in a self-sustaining chain reaction that occurs very quickly,
after it begins.
As with most things in nature, the results
of activation energies can be either bad or good. We want some reactions
to occur, but they don't occur due to activation energies, and this is
bad. But gasoline doesn't burn in a car's gas tank, only in the engine,
and this is good. Activation energies are also biologically useful
because they provide a kinetic obstacle — so undesirable reactions
are prevented, and (as explained below) desirable reactions can be controlled — and
this allows life.
How our
bodies make unusual things happen.
Inside our bodies, reactions
occur that would not occur outside our bodies, and molecules exist
that would not exist outside our bodies. How and why can
this happen?
kinetics: Many reactions that
usually are kinetically unfavorable can occur because some proteins,
which are called enzymes, act as catalysts that "make
things happen" by providing a way to lower the activation energy and/or
bring chemicals together in a spatial orientation that is "just right" for
reacting. Enzymes operate in the context of control
systems that control which reactions do and don't occur, and when. These
control systems are analogous to a thermostat that turns a furnace on and
off, when we do and don't need heat, but are much more complex and wonderful.
thermodynamics: Many reactions
that usually are thermodynamically unfavorable can occur when they are
part of a coupled reaction. For example, if a biologically useful
reaction that is unfavorable (because it produces a change of -400 in universe-entropy)
is combined with a sufficiently favorable reaction (that produces a change
of +500 in universe-entropy) the overall coupled
reaction is favorable, since it produces an increase of +100 in
universe-entropy. Our bodies use external fuel (with chemical potential
energy stored in the foods we eat and the oxygen we breathe) to produce
internal fuel (with energy temporarily stored in "high energy" molecules
such as adenosine triphosphate, ATP) that is used in coupled reactions.
Converting Energy into Useful
Functions
The overall result of biochemistry (the
chemistry that occurs inside our bodies) is to produce a "living environment" with
molecules and reactions that would not occur outside our bodies. This is
a metastable environment, with high energy and low entropy, that is maintained
by the use of external energy. In a book about The Mystery of Life's
Origin, Chapter 7 (the
first chapter about thermodynamics) explains how living organisms can exist
despite their unfavorable energy and entropy, by converting external energy into
useful internal functions. Here is my brief summary:
Localized areas of high entropy can be maintained
by a flow of energy. For example, if electrical energy flows through
water, HH will form at one electrode and OO will form at the other electrode,
reversing the favorable "explosive reaction to form HOH" so it becomes
the normally unfavorable "HOH + HOH --> HH + OO + HH". Or,
supplying energy to a refrigerator can lower the temperature inside it, even
though this would never occur (and it would violate the Second Law) under ordinary
circumstances. But as long as energy continues to flow through the refrigerator,
and its "mechanism that produces cold" is operating properly, the
high-entropy cold area can be maintained, in a way that is consistent with
the Second Law. Similarly, energy flow through our bodies can — due
to the mechanisms that maintain life, that convert food energy into biologically
useful functions — maintain our bodies in a high-entropy state.
The book's authors (Charles Thaxton
and Walter Bradley, plus geologist Roger Olsen) explain why a "coupling
mechanism" is necessary: "Maintenance
of the complex, high-energy condition associated with life is not possible
apart from a continuous source of energy. A source of energy alone
is not sufficient, however, to explain the origin or maintenance of living
systems. The additional crucial factor is a means of converting this
energy into the necessary useful work to build and maintain complex living
systems from the simple biomonomers that constitute their molecular building
blocks. An automobile with an internal combustion engine, transmission,
and drive chain provides the necessary mechanism for converting the energy
in gasoline into comfortable transportation. Without such an "energy
converter," however, obtaining transportation from gasoline would
be impossible. In a similar way, food would do little for a man whose
stomach, intestines, liver, or pancreas were removed. Without these,
he would surely die even though he continued to eat. Apart from a
mechanism to couple the available energy to the necessary work, high-energy
biomass is insufficient to sustain a living system far from equilibrium. In
the case of living systems such a coupling mechanism channels
the energy along specific chemical pathways to accomplish a very specific
type of work. We therefore conclude that, given the availability
of energy and an appropriate coupling mechanism, the maintenance of a living
system far from equilibrium presents no thermodynamic problems."
The authors
also emphasize the important difference between producing a
coupling mechanism (during chemical evolution to
produce the first generation of life) and maintaining it
(during many succeeding generations of life, to allow a process of biological
evolution): "While the maintenance of
living systems is easily rationalized in terms of thermodynamics, the origin of
such living systems is quite another matter."
During biological evolution after life
is established and stable, the basic life-allowing mechanisms already
exist, are inherited with sufficient reliability through many generations,
and in principle these basic mechanisms can increase in variety and complexity
through a neo-Darwinian ratchet process so the
relevant questions are "What types of complexity
can be produced, and how quickly?" But in chemical evolution, the
basic mechanisms do not exist (so life cannot exist) but they must
be produced (so life can exist), and this seems much more challenging. |
This website for Whole-Person Education has TWO KINDS OF LINKS:
an ITALICIZED LINK keeps you inside a page, moving you to
another part of it, and
a NON-ITALICIZED LINK opens another page. Both keep everything inside this window,
so your browser's BACK-button will always take you back to where you were.
|
Here are other related pages:
This page, written by Craig Rusbult, is
http://www.asa3.org/ASA/education/origins/thermo-life.htm
Copyright © 2005 by Craig Rusbult
all rights reserved
 The
ball is thermodynamically unstable because
it would roll down the hill if it could. But this thermodynamically
favorable reaction will occur only if the ball can first escape from the
bowl. The action of the ball climbing up and over the bowl's edge
is analogous to chemicals overcoming their activation energy. Until
this occurs, the ball is in a metastable state;
it is temporarily stable, even though it would react (by rolling down the
hill) if it could. Similarly, the gasoline and oxygen would react,
but they don't react until the spark allows some of the molecules to overcome
their activation energy; this lets them react and they "release
energy" to their neighbors, which lets these neighbors overcome their
activation energy so they can react and release energy to their neighbors,
and so on, in a self-sustaining chain reaction that occurs very quickly,
after it begins.
The
ball is thermodynamically unstable because
it would roll down the hill if it could. But this thermodynamically
favorable reaction will occur only if the ball can first escape from the
bowl. The action of the ball climbing up and over the bowl's edge
is analogous to chemicals overcoming their activation energy. Until
this occurs, the ball is in a metastable state;
it is temporarily stable, even though it would react (by rolling down the
hill) if it could. Similarly, the gasoline and oxygen would react,
but they don't react until the spark allows some of the molecules to overcome
their activation energy; this lets them react and they "release
energy" to their neighbors, which lets these neighbors overcome their
activation energy so they can react and release energy to their neighbors,
and so on, in a self-sustaining chain reaction that occurs very quickly,
after it begins.