

Creation/ Evolution Page
Transitional Forms and the Evolution of Phyla
Glenn R. Morton*
Ramsden House
glenn.morton@btinternet.com
105 Malcolm Road
Peterculter, Aberdeen AB14 0XN Scotland
From: Perspectives on
Science and Christian Faith 53.1 (March 2001): 42-51.
The claim is often made in Christian circles that there is no evidence for phylum level evolution. Evidence, in the form of morphological similarities, is presented showing that transitional forms connecting phyla do exist. Specific morphological connections are examined which unite the lobopods, arthropods, brachiopods, molluscs, and annelids. By examining these lineages, evidence arises indicating that the Cambrian Explosion was not very explosive. In contradiction to many apologetical claims, it occupied a period of nearly 100 million years. This paper discusses the causes of the rapid differentiation and apparent abundance of Cambrian animals, adding to the evidence that the Cambrian does not represent the creation event. For some Christians to equate the Cambrian Explosion with the creation event ignores the massive evidence of animals appearing gradually beginning in the Precambrian and continuing into the Cambrian. The creation of life on earth must be much earlier than the early Cambrian.
One of the most common claims among Christian apologists, of both the young-earth and old-earth schools, is the claim that there is no evidence for macro-evolution, or evolution between the phyla. Duane Gish, a young-earth creationist, presents that position when he writes:
Evolutionary theory is, of course, dead, as long as the two huge gaps between single-celled organisms and the complex invertebrates and between complex invertebrates and fishes continue to exist. The total failure to reduce these gaps, let alone close them, in spite of an intense search by thousand of paleontologists during more than 125 years, establishes beyond doubt that the required transitional forms will never be found. The fact that the gaps between all higher taxa, such as families, orders, classes, and phyla, are systematic and almost always large, is simply additional confirmatory evidence for creation.1
Ray Bohlin writes in a recently released book:
The origin of the different types of invertebrate animals such as the sponges, mollusks, echinoderms like the starfish, arthropods like crustaceans, and others all appear suddenly, without ancestors, in the Cambrian period.2
Other authors, like Davis and Kenyon,3 Vos,4 Wise,5 and Johnson,6 say essentially the same thing. One would think that with such near unanimous agreement that there could be no doubt about the lack of transitional animals during the Precambrian/Cambrian transition when the major phyla appeared on earth. However, data developed over the last five years have clearly demonstrated that there are transitional fossils between the major phyla.
We intend to demonstrate that (1) the phyla are not morphologically isolated from other phyla, (2) there are transitional forms, (3) the Cambrian Explosion, far from being a record of the instantaneous creation of life, was part of a 100-million-year long gradual development of diversity, and (4) the causes of this increase in diversity are consistent with natural causes rather than Divine.
Definitions
One must define what phyla is before one can know if there are transitional forms between them. A phylum is often defined as a major body plan type, a bauplan. Vertebrates share many traits, among them a spinal cord enclosed in a vertebral column. Mollusks do not have a backbone or spinal cord. Thus they are not vertebrates. The number of phyla varies from taxonomist to taxonomist but generally hovers around thirty to thirty-five.7
Phyla are not the absolutely static categories that many apologists think. In fact, the use of phyla as static categories depends upon a circularity that most apologists seem not to recognize. An animal found either on earth today or in the fossil record must be placed into a phylum by the taxonomist. Taxonomists are reluctant to define a new phylum for a specimen which does not fit into any of the existing phyla. Because of this, there is a tendency to shoehorn problematical fossils into one of the existing phyla. Thus, one cannot say that because the animal was not placed in a "transitional" phylum, that there are no transitional forms. Since many problematical fossils that give evidence of either incipient phyla or transitions between the phyla have been difficult to classify, they have been placed in different phyla by different authors. Wiwaxia, an animal we will examine below, has been classified both with the annelids and with the mollusks, and indeed shares traits with both groups.
An example of a possible modern incipient phylum concerns a deep-sea sponge found recently. It is classified among the Porifera, an existing phyla, in spite of it having a unique body plan. Vacelet and Boury-Esnault write:
Our results raise fundamental questions about the validity of characteristics used to distinguish the phyla of lower invertebrates. A sponge is defined as a "sedentary, filter-feeding metazoan which utilizes a single layer of flagellated cells (choanocytes) to pump a unidirectional water current through its body." Except for being sedentary, the cave Asbestopluma and presumably all Cladorhizidae lack these basic sponge attributes. In an extreme environment where active filter-feeding has a low yield, cladorhizids have developed a mode of life roughly similar to that of foraminiferans or cnidarians. Their feeding mechanism relies on passive capture of living prey and on transfer of nutrients into the body through intense cell migrations, the analogue of cytoplasmic streaming in foraminiferan pseudopodia. This may be compared to the emergence of macrophagy in abyssal tunicates, also accompanied by a reduction of the filtering system although in Cladorhizidae the result is more extreme, with a main body plan different from Porifera and resembling no other modern anatomical design.
Such a unique body plan would deserve recognition as a distinct phylum, if these animals were not so evidently close relatives of Porifera. Their siliceous spicules show clear similarities to several families of poecilosclerid Demospongiae.8
Such a shoehorning of the Cladorhizidae into Phylum Porifera hides the obvious transitional nature of this creature. The morphological connection between it and the sponges is the spicules. Were they to be lost during subsequent evolution, one would have little reason to place this animal with the sponges. However, anti-evolutionists can currently claim that there are no intermediates among the phyla, simply because this incipient phylum was placed into its parent phylum.
We need to define what a transitional form is. Only one anti-evolutionary author I have read, who speaks about transitional forms, bothers to define the term.9 A transitional form is one that shares traits between two or more phyla. As noted above, there is a tendency to place a form into one or another taxon. This hides the fact that a given form shares traits with another group. Therefore, when evaluating the claim that a specimen represents a transitional form, one cannot use the fact that it has been placed in one taxa rather than in an intermediate one. It is very difficult to classify such an animal because it does not quite fit the definition of either group. Thus, it is simply not valid to insist that the animal be classified in one or the other predefined group and then claim that there are no transitional forms.
Transitional forms are not transitional between modern living members of two phyla but are transitional between the two phyla when they were evolved.
Due to the vagaries of fossilization, it is not expected that we will ever find animals which are on the direct line between the two groups, and if we do find an animal on the direct line, it is not clear that we would recognize it as such. Rather what we have in the fossil record are animals that are close to the line of descent. Nevertheless even these animals will show many of the traits that were involved in the actual line of descent between the two phyla. Even if we found the individual animal that is on the direct line of descent between two groups, we would not have any way of knowing it. We must treat all transitional forms as if they are near to the actual line of descent. One cannot determine what a transitional form should look like by examining modern forms. This is as useless as trying to tell what your grandfather looked like by averaging your looks and your cousin's looks. It simply will not work. Finally, in evaluating a potential transitional form, it must be understood as being transitional between the phyla as they existed at or near the time of the their appearance. Transitional forms are not transitional between modern living members of two phyla but are transitional between the two phyla when they were evolved.
One other feature of phyla needs to be noted. The phyla were defined over a century ago based on extant (living) animals. Subsequently, all fossil creatures were placed into present phyla. This is like trying to classify the parts of a tree by fitting every part into a classification derived from only examining the very tips of the branches--hardly a satisfactory way of doing things. Just as the branches of a tree arose from the roots and trunk, modern phyla arose from ancient life, not vice versa.
The Cambrian/Precambrian boundary has often been used by apologists as the point in time when God created life. In 1979, Gish wrote:
Not a single, indisputable, multicellular fossil has ever been found in pre-Cambrian rocks! Certainly it can be said without fear of contradiction that the evolutionary ancestors of the Cambrian fauna, if they ever existed, have never been found.10
This was not true then and is not true today. The plain fact is that Precambrian multicellular fossils were found in 1947 by R. C. Sprigg. He classed some of them among the worms, sponges, and jellyfish, making them clearly ancestral to the Cambrian creatures.11 This discovery occurred twenty-two years before Gish wrote that erroneous statement.
DeHaan and Wiester make the same error as Gish in claiming:
The Cambrian explosion refers to the geologically sudden appearance of multicellular animals during the geologic period called the Cambrian.12
Indeed, multicellularity appears in the fossil record 1.7 billion years ago, long prior to when these gentlemen claim and long prior to the Cambrian explosion.13 The emergence of multicellularity had nothing to do with the Cambrian explosion.
Other authors, such as Robinson,14 Davis,15 and Johnson,16 while apparently not mistaking the Cambrian explosion with the appearance of multicellularity, do claim that the Cambrian animals appeared out of nowhere. Such views of the fossil record are simply false. The Cambrian/Precambrian boundary is no longer viewed as marking an instantaneous appearance of life or multicellularity. Grotzinger, et al. write:
Once held as the position in the rock record where the major invertebrate groups first appeared, the Precambrian-Cambrian boundary now serves more as a convenient reference point within an evolutionary continuum. Skeletalized organisms, including Cambrian-aspect shelly fossils, first appear below reference point within an evolutionary boundary and then show strong diversification during the Early Cambrian. Similarly, trace fossils also appear first in the Vendian, exhibit a progression to more complex geometries across the boundary, and then parallel the dramatic radiation displayed by body fossils.17
Among the phyla now found from the latest Precambrian are Porifera (sponges),18 Mollusca,19 Annelida,20 Cnidaria,21 and Arthropoda.22 And thirteen phyla appear after the Cambrian. These are Bryozoa, Angiosperms, dinoflagellates, diatoms, nannoplankton, bryophytes, ferns, ginkgoes, psilophytes, sphenopsids, cycadeoids, lycopods, and conifers. The Cambrian "explosion" is getting less explosive. It is not the place in the geologic column where God created life. That occurred much earlier with the creation of the first cell.
The Fossil Record
As seen in Table 1 (compiled from various sources), the earliest accepted worm burrows are found in rocks dated to 680 million years ago. They are horizontal and shallow in orientation as if the worm was scavenging the organic material in the shallowest sediments. There have been controversial reports of worm burrows as far back as one billion years ago. In any event, worms appear long before the Cambrian, and this fact falsifies several recent
|
Table 1. Cambrian Chronology (Except where otherwise noted, data from ref. 55) Million Years Ago (myr) Event 495 End of Cambrian |
statements by apologists. At Doushantuo, China, larvae of segmented worms have been found dating between 570-520 myr ago.23
Because worm-like animals appear to be the earliest active, multicellular animals, it is reasonable to believe that they are the evolutionary ancestors of all phyla. Are there any connecting links between the worms and other phyla? It appears that there are. A fossil lobopod, Aysheaia pedunculata is basically a worm with "legs," or more properly, lobopods. The word lobopod is used in two ways: to reference a particular kind of animal leg and to refer to a group of animals possessing such legs. The lobopods consist of muscles surrounding a blood- filled cavity. The lobopods are soft and pliable, and are used as organs of locomotion. Aysheaia has spikes on its legs that are used to grasp prey. Aysheaia is a member of the phylum, Lobopoda.24 While there is nothing that looks like an arthropod in Aysheaia, the lobopods will be used to show a connection to the arthropods through something like the next animal, Anomalocaris.
The Arthropods
Anomalocaris was first described by Charles D. Walcott in 1912,25 contrary to what one recent apologetical book claims.26 Unfortunately, the only feature described was the claw of an arthropod. Anomalocaris had lots of claws. It was not until 1985 that the entire animal was known.27 Anomalocaris was a meter long predator of the Cambrian seas.
By 1998 specimens were known that showed more features. Anomalocaris, the animal with an arthropod claw, also had lobopod legs.28 Other early Cambrian arthropod relatives, like Opabinia, also were discovered to have lobopod legs.29 In addition Anomalocaris had other traits connecting it to arthropods. It had a tail fan and flap-like projections that perform several purposes: protection, propulsion, and respiration. Conway Morris suggests:
Of these, perhaps the last was the most important. Imagine that in due the flaps seen in Anomalocaris were modified. The leading edge now formed an elongate bar and behind it the structure was transformed in a series of trailing filaments. This, of course, would be reminiscent of the gills that arise above the walking legs of many arthropods. Thus, if the lobopod limbs were transformed into jointed appendages and the flaps into gills, then one could envisage, at least in broad outline, the transformation between an animal similar to Anomalocaris and a fully fledged arthropod.37
Thus, these early arthropods, like Anomalocaris, are transitional between the lobopods and the arthropods.
Anomalocaris also has one trait that connects it further back to the worms. Its mouth is worm-like. Collins notes of the Anomalocarids:
[their] "circum-oral sclerites"[radiating teeth] indicate that the anomalocarids "were related to aschelminth worms rather than to arthropods." Studying other specimens of the same Chengjiang species, Ramskold described the abdominal appendages in greater detail as "three or more [postoral] limb pairs modified into large gnathobases which form part of the masticatory apparatus," and "trunk limbs" with "small, jointed clawed endopods" and "exopods ... modified into large lateral flaps."38
Having some traits in common with worms and lobopods is exactly what one would expect of a transitional form. What we see in the fossil record is first worms, then lobopods, then animals with lobopod and arthropod appendages with a worm-like mouth and possible incipient arthropod gills. Finally, we see the true arthropods. This is a transitional series.
Trilobites, contrary to popular
understanding of the fossil record, do not appear at the beginning of the
Cambrian. They appear in the Atdabanian stage, 15 million years after
the beginning of the Cambrian.39 Nor do they spring
out of nowhere. Precambrian precursors to the trilobites include Spriggina
and Bomakellia. Bomakellia and a trilobite can be seen in
Figure 1 and 2. Obviously the claim that there are no possible precursors to the
Cambrian animals is simply wrong.40
The Brachiopods
When we turn to the brachiopod phylum we also find an interesting precursor which is never discussed in apologetical literature. The 525-myr-old animal, Halkieria evangelista, from the Sirius Passet formation of Greenland, is a perfect transitional form between worms and the brachiopods. The animal has a flattened body with a soft, rubbery underside, like a slug. It moved across the ocean floor by means of this slug-like bottom. Its upper surface was protected by scales, called sclerites, which were hollow and had a stem which was inserted into the back making a chain-mail type of armor. The scales were arranged in three broad zones with a different shaped scale in each zone. The sides of the animal were covered with knife-shaped scales, the edges of the belly had sickle-shaped scales, and the back had palm-shaped scales. These three zones of scales will become important when we discuss the origin of the annelid (worm) phylum.41
Fig. 1 Cambrian Tribolite
One curious feature of the Halkieria
is the fact that there was a shell at each end of the animal, on the back side.
The posterior shell was nearly identical to the shells of the earliest Cambrian
brachiopods, like Obolella.42 It is
postulated that when attacked, Halkieria curled up between the two
shells for protection.43 As predation increased,
eventually the animal remained between the two shells and took up a sessile
life. There is one very primitive  brachiopod today, Neocrania, which
early in life does crawl across the ocean bottom as the halkieriids did.44
Eventually, as it matures, Neocrania folds itself in two and secretes a
shell, like other brachiopods.
brachiopod today, Neocrania, which
early in life does crawl across the ocean bottom as the halkieriids did.44
Eventually, as it matures, Neocrania folds itself in two and secretes a
shell, like other brachiopods.
Even among extant members of different phyla, there are often strikingly complex, individual features that both possess which tie them together as descendants of common ancestral taxa. For example, brachiopods have chitinous bristles that protrude into the surrounding water from each of the valves. The microscopic texture of these bristles is identical to that of the bristles of the annelid worms. The hypothesis that God
Fig 2. Precambrian Bomakellia
created all the animals at the Cambrian/Precambrian boundary fails to account for this similarity. But the place of Halkieria in the evolution of both the brachiopod phylum and the annelid phylum explains this similarity.
The Molluscs
Over the past three years, new information has come to light concerning a possible relative of the halkieriids, which also sheds light on the origin of mollusks. Kimberella is a 555-myr-old Precambrian creature, which was bilaterally symmetric. It is known from the White Sea region of Russia and Australia. Kimberella had a strong nonmineralized shell and crawled along the water bottom on a muscular slug-like foot.45 While no specimens show signs of the characteristic mollusc feeding apparatus, the radula, radula markings have been found in strata containing this possible mollusc ancestor. It is not surprising that the radula is not found with Kimberella, as it is not found with most mollusc fossils.46 All of this information strongly implies that mollusc ancestors were alive prior to the Cambrian explosion at 545 myr ago.
Over the past three years, new information has come to light concerning a possible relative of the halkieriids, which also sheds light on the origin of mollusks.
Like Kimberella, Halkieria possesses bilateral symmetry and a soft-sole underside. It, too, is believed to have been an algae grazer.47 Besides giving rise to the phylum of the brachiopods, Halkieria appears to be close to the lineage leading to two other phyla, the molluscs and polychaete worms through a related animal similar to Wiwaxia (although Wiwaxia itself lived too late to be the actual ancestor). Wiwaxia is connected to the halkieriids by many traits. Like the halkieriids, it had a soft slug-like sole upon which it moved, and sclerites that possessed identical microscopic structure and were arranged in the same three zones as seen on the halkieriids. In both Halkieria and Wiwaxia, the sclerites were hollow. Many paleontologists believe that these connections prove a close relationship.
But Wiwaxia takes things one step further. It is the earliest known animal to possess the radula, the characteristic feeding mechanism of the molluscs.48 Wiwaxia shows other connections with the most primitive molluscs, the aplacophorans. To quote Conway Morris:
It is generally accepted that the aplacophorans, a rather obscure group well understood only by a handful of specialists, are our best glimpse of the likely appearance of the most primitive molluscs. These animals look like some sort of spiny worm because, in contrast to other molluscs, which have a shell of some sort, in the aplacophorans the surface is coated with numerous calcareous spicules. Many zoologists believe that the shells of the other molluscs originated by the fusion of these spicules.49
Thus, an animal similar to Wiwaxia, could easily have fused its scales to form the shells of the earliest molluscs.
The Annelids
Wiwaxia appears to shed some light also on the origin of the annelid worm phylum. The polychaete annelid worms are spiny or hairy looking creatures. The "hairs" are called chaetae, and they are mineralized. The microscopic structure of the wiwaxian scales is identical to that of the notochaetae of the annelids showing a connection between the two.50
One other connection between these two creatures, and the halkieriids, firms up the suggested phylogeny. At least one specimen of Wiwaxia (USNM199936) had a structure on one end resembling a brachiopod shell, which Conway Morris and Peel interpreted as a vestigial shell.51 This is reminiscent of Halkieria's posterior shell and connects Wiwaxia with Halkieria. But the polychaete annelid, Canadia, shows that it also has a posterior plate made of chitin, called a sternaspid, continuing the similarity in this chain. Conway Morris and Peel state:
In any event, the similarities of the shield with the posterior halkieriid shell may not be convergent, and the possibility of sternaspids being primitive should be investigated. In particular, although the evidence is slender there is quite a strong similarity between the sternaspid plate and the putative shell in Wiwaxia.52
Once one sees these connections, one simply cannot return to the blithe statements so often seen in the apologetical literature, like that of Rana who wrote:
Fossils previously found in Yunnan province (at sites discovered nearly 100 years ago) and in the Burgess Shale deposits of the Canadian Rockies tell us that all animal phyla (more than 70) ever to exist in Earth's history appeared "at once" about 540 million years ago. (Some 40 phyla have since disappeared and not a single new one has appeared.)53
Such statements display the fact that the author has neither studied the data concerning the history of life since the Cambrian nor the paleontological evidence from the Cambrian/Precambrian boundary. Knowledge of this time period has improved tremendously over the past fifty years. Lazarus J. Salop, in 1983, wrote:
Progress in Precambrian geology has been exceptionally great, indeed, quite striking for geologists of the older generation; only some 30-40 years ago the Precambrian appeared as an uncertain and even mystic prelude to geologic evolution. Even the very name-Precambrian-means some indivisible unit in the early history of the Earth, the beginning of which is poorly known.54
The knowledge gain has accelerated exponentially since Salop wrote that, but when it concerns the Cambrian explosion, apologists remain stuck in the world of fifty years ago.
What Caused the Cambrian Explosion?
There are several schools of thought on the cause of the Cambrian explosion. We will look at some of them. The first is the most widely held view among the Christian community. It is that God created the living creatures in a geological instant of time early in the Cambrian. As we have seen, the animals do not all appear at the same time within the Cambrian. Many animals and phyla appear before and after the Cambrian. Thus, this view is contradicted by the data at hand. Does this mean that God did not create the animals? No. It does mean that God did not do it in one blinding flash of creativity.
One basic fact explains the supposed "sudden" appearance of animals in Cambrian strata. It is the appearance of hard shells on animals where none had existed before. This imparted a much greater possibility for fossilization than had existed before. Soft flesh does not fossilize easily but hard shells do. Various theories have been advanced to account for the widespread acquisition of hard skeletons among the taxa during the Cambrian. Probably a synergistic combination of the various factors emphasized by these different theories acted to effect the evolutionary change. We will briefly look at four theories: the detox theory, the biolocomotory theory, the predation theory, and the biogeographic theory.
The detox theory of the Cambrian explosion postulates that organisms deposited excess phosphorus in external shells under selection pressure to rid themselves of its toxic effect. Conway Morris writes:
It is certainly not immediately clear how this break-up of the super-continent might change the balance and distribution of food supply in the oceans. But perhaps there are some clues. The interval marked by the Cambrian "explosion" is also a time when quite unusual amounts of sedimentary phosphate were being deposited in the shallow shelf seas that rimmed continents. These regions are now found in such places as Australia, South China, and Kazakhstan. Precise estimates of the total volume of phosphate that accumulated in the geological interval are not easy to obtain. Nevertheless, the fact that some of the world's most important mines that extract this phosphate, largely to provide agricultural fertilizer, are situated in rocks of Cambrian age gives a crude indication of the massive quantities of phosphorus that must have been deposited.55
One basic fact explains the supposed "sudden" appearance of animals in Cambrian strata. It is the appearance of hard shells on animals where none had existed before.
The detox theory postulates that the organisms in their attempts to rid themselves of phosphorous, deposited it in external shells. This view is supported by the unique make up of Cambrian exoskeletons. Lipps and Signor write:
Some Cambrian arthropods had cuticles which were unique in the history of the phylum: they were composed of calcium phosphate. This is of particular interest in view of the common occurrence of phosphatic shells in other phyla in the Cambrian. There are no recent arthropods with a cuticle composed entirely of calcium phosphate, although a phosphatic component is present in some cuticles (e.g., the crab Cancer), especially in their outer part.56
Robert Carroll relates variations on the theme of skeletons forming because of the metabolic handling of calcium and phosphorous. He states:
Tarlo argued that bone originally developed not for protection, but as a phosphate reserve. Phosphate is both a vital component for energy storage and transfer in all vertebrates and a substance that may be available in fluctuating amounts in the natural environment. The amount of phosphate is frequently a limiting factor in population growth. Superficial bone in some Paleozoic fish shows changes in its extent that are thought to be seasonal and may correspond to the periodic deposition and resorption of phosphate. Bone also acts as a calcium reserve in all vertebrates.57
The biomechanical locomotor theory simply takes note of the fact that muscles can be more efficient if they have something against which to exert their force. Without skeletons, animals would have moved slowly in a wormlike fashion. Skeletons allowed animals to move more rapidly and thus provided the selective pressure to favor skeletal development.
The predation theory is the oldest of the views and was first proposed by Albert Gaudry in 1883. This view advocates that the rise of predation provided a selective pressure in favor of defensive protection. He wrote:
The majority of animals found in the Paleozoic, and especially in the Silurian, appear to have been better suited to defense than to attack, as if, in the early days of the world, these creatures (which are rare today) had had a greater need to be protected.58
This view is supported by the lack of evidence for predation until the very latest Precambrian. Boreholes, drilled into the calcified tubes of Cloudina, are the first unambiguous evidence of predatory behavior.59
From this time on, animals seemed to change their morphology and behavior in manners consistent with the idea that they were trying to avoid predators. Worm burrows, which had been shallow and horizontal changed to vertical as the animals buried themselves in the ocean floor as if to escape possible predators.60 Other animals built "stone walls" or shells around themselves. The shells rapidly grew thick across many taxa allowing the animals to survive. This massive investment in defensive armor had the unintentional by-product of allowing animals to become fossilized. It is this fossilization that led earlier researchers to remark on the "sudden" appearance of animals--an appearance which is now known to be a progressive appearance of more and more complex animal life forms extending from 700 million years ago to 500 million years. And it was not just metazoans that became skeletonized at this time. Apparently microscopic foraminifera became skeletonized, also. Culver argues that this fact is only consistent with predation as a cause of the sudden skeletalization of major parts of the biosphere. He writes:
Discussions concerning the appearance of skeletonization near the base of the Cambrian [about 550 Ma (million years ago)] are often restricted to metazoans and take little account of the acquisition of hard parts by protists at the same time. For example, hypotheses relating the evolution of skeletonization to increases in body size and to detoxification of excess calcium in metazoans do not apply to protists and hence are weakened by the appearance of testate protists in the Early Cambrian. However, this appearance is not inconsistent with the hypothesis, applicable to both metazoans and protists, that the initial function of skeletons was to protect the organism, primarily against predation. The presence of agglutinated foraminifera in the Lower Cambrian, probably Atdabanian Stage-equivalent strata, of the Taoudeni Basin, West Africa is reported here. These specimens extend considerably the known geologic range of several genera, they represent the earliest known unequivocal foraminifera, and they further remind us that protists as well as metazoans should be considered in accounting for the origin of skeletalization.61
The biogeographic theory revolves around geologic changes that occurred during the last stage of the Precambrian. A global continent, called Rodinia, began to rift and break apart. As Hagadorn and Waggoner state:
Rifting of the supercontinent Rodinia occurred in the late Proterozoic, separating the Cordilleran margin of Laurentia from east Gondwanaland. This rifting may have begun 150-200 million years before the Cambrian, or possibly much later, in the Vendian.62
The change of environment caused by this event may have played a role in the Cambrian explosion, causing animals to exploit new habitats or as noted above, causing an influx of phosphorous to the oceans requiring animals to excrete it in the form of exoskeletons
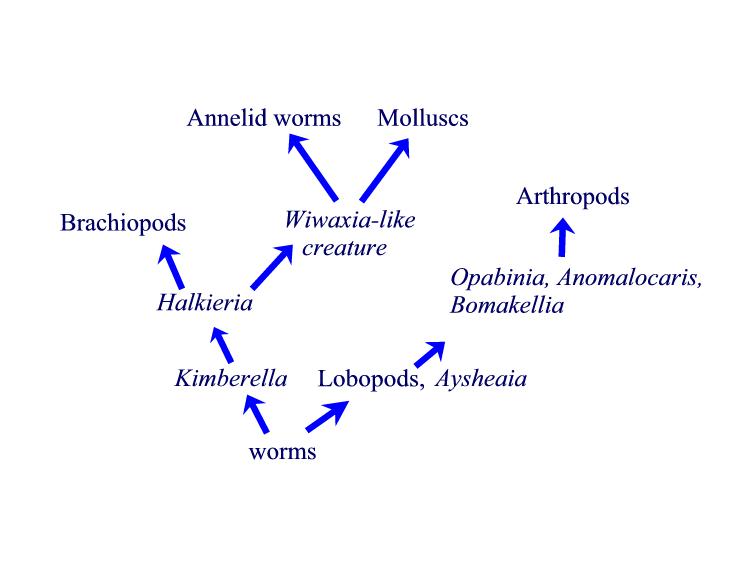
Fig 3. Phyla level transitions. Transitional forms (italic
font) are placed between the phyla (Roman font).
Suggested evolutionary relationships marked by arrows.
Conclusion
Figure 3 shows the relationships between the various transitional forms we have discussed. The transitional forms (italics) are placed between the phyla (Roman text) that they connect. The morphological connections we have seen clearly show that phyla level transitional forms exist. Apologists have an obligation to include these forms in their apologetical views. Furthermore, the Cambrian Explosion cannot be equated with the creation event. As we have seen above, the diversification of animals began in the late Precambrian and continued into the Cambrian. The entire period of this diversification took over fifty million years. Indeed, worm tracks are found 120 million years prior to the Cambrian. It is true that the most rapid part of the diversification took around ten million years, but that ten million years was part of a longer period of diversification. It is inconsistent for Christians to pay attention to one part of this period as if it were the creation event, when, in fact, species were appearing over a much longer period of time. It is time for Christian apologists to catch up with the information from the last century.
Notes
1 Duane T. Gish, Creation Scientists Answer their Critics, (El Cajon: Institute for Creation Research, 1993), 135.
2 Ray Bohlin, "The Natural Limits to Biological Change," in Ray Bohlin ed., Creation, Evolution & Modern Science (Grand Rapids: Kregel Publications, 2000), 41.
3 Percival Davis and Dean H. Kenyon, Of Pandas and People, (Dallas: Haughton Publishing Co., 1993), 94.
4 Howard Vos, Genesis (Chicago: Moody, 1982), 20.
5 Kurt P. Wise, "The Origin of Life's Major Groups," in J. P. Moreland, ed. The Creation Hypothesis (Downer's Grove: InterVarsity Press, 1994), 226.
6 Philip Johnson, Darwin on Trial (Downer's Grove, InterVarsity Press, 1991), 54.
7 Scott F. Gilbert, Developmental Biology, 3d ed. (Sunderland, MA: Sinauer Assoc., 1991), 831.
8 J. Vacelet and N. Boury-Esnault, "Carnivorous Sponges," Nature 373 (1995): 335.
9 Leonard Brand, Faith Reason and Earth History (Berrien Springs: Andrews University Press, 1997), 147.
10Duane Gish, Evolution: The Fossils Say No (San Diego: Creation-Life Publishers, 1979), 79-80.
11Gonzalo Vidal, "The Oldest Eukaryotic Cells," Scientific American 250, no. 2 (1984): 48.
12Robert F. DeHaan and John L. Wiester, "The Cambrian Explosion," Touchstone (July/August 1999): 66.
13Zhu Shixing and Chen Huineng, "Megascopic Multicellular Organisms from the 1700-Million-Year-Old Tuanshanzi Formation in the Jixian Area, North China," Science 270 (1995): 620.
14Steven J. Robinson, "From Flood to Pharaoh--A Chronological Framework," The Genesis Agendum, Occasional Papers 2 (1998): 3.
15John Jefferson Davis, "Response to Howard Van Till" in J. P. Moreland and John Mark Reynolds, eds., Three Views on Creation and Evolution (Grand Rapids: Zondervan, 1999), 229.
16Phillip E. Johnson, Defeating Darwinism (Downers Grove: InterVarsity Press, 1997), 95.
17John P. Grotzinger, Samuel A. Bowring, Beverly Z. Saylor, and Alan J. Kaufman, "Biostratigraphic and Geochronologic Constraints on Early Animal Evolution," Science 270 (1995): 603-4.
18Martin Brasier, Owen Green, and Graham Shields, "Ediacaran Sponge Spicule Clusters from Southwestern Mongolia and the Origins of the Cambrian Fauna," Geology 25, no. 4 (1997): 303.
19Mikhail A. Fedonkin and Benjamin M. Waggoner, "The Late Precambrian Fossil Kimberella is a Mollusc-like Bilaterian Organism," Nature 388 (1997): 868.
20Preston Cloud and Martin F. Glaessner,"The Ediacarian Period and System: Metazoa Inherit the Earth," Science 217 (1982): 788.
21Simon Conway Morris, The Crucible of Creation (Oxford: Oxford University Press, 1998), 29.
22Benjamin M. Waggoner, "Phylogenetic Hypotheses of the Relationships of Arthropods to Precambrian and Cambrian Problematic Fossil Taxa," Systematic Biology 45, no. 2 (1996): 190.
23Stefan Bengtson and Yue Zhao, "Fossilized Metazoan Embryos from the Earliest Cambrian," Science 277 (1997): 1645.
24R. A. Robison, "Affinities of Aysheaia (Onychophora), with Description of a New Cambrian Species," Journal of Paleontology 59 (1985): 227.
25C. D. Walcott, "Middle Cambrian Branchipods, Malacostraca, Trilobita and Merostomata," Cambrian Geology and Paleontology II, Smithsonian Miscellaneous Collections 57 (1912): 145-228.
26Ray Bohlin, "Evolution's Big Bang," in Ray Bohlin, ed., Creation, Evolution, & Modern Science, (Grand Rapids: Kregel Publications, 2000), 49.
27H. B. Whittington and D. E. G. Briggs, Philosophical Transactions of the Royal Society of London, B, 309 (1985): 569-609.
28Conway Morris, 183.
29G. E. Budd, "The Morphology of Opabinia regalis and the Reconstruction of the Arthropod Stem-group," Lethaia, 29 (1996): 13; Pambdelurion another anomalocarid from the Cambrian seas also has lobopod legs. See Graham E. Budd, "Arthropod Body-Plan Evolution in the Cambrian with an Example from Anomalocaridid Muscle," Lethaia 31 (1998): 197-210.
30T. Peter Crimes, "Trace Fossils and Correlation of Late Precambrian and Early Cambrian Strata," Geology 124 (1987): 107, 108, 109, 110, 111.
31Stephen J. Culver, "Early Cambrian Foraminifera from West Africa," Science 254 (1991): 689.
32M. W. Martin, et al., "Age of Neoproterozoic Bilaterian Body and Trace Fossils, White Sea, Russia: Implications for Metazoan Evolution," Science 288 (2000): 841-5.
33James G. Gehling and J. Keith Rigby, "Long Expected Sponges from the Neoproterozoic Ediacara Fauna of South Australia," Journal of Paleontology 70, no. 2 (1996): 185; and Chia-Wei Li, Jun-Yuan Chen and Tzu-En Hua, "Precambrian Sponges with Cellular Structures," Science 279 (1998): 879.
34Martin, et al., "Age of Neoproterozoic Bilaterian Body and Trace Fossils."
35Mark Pagel, "Inferring the Historical Patterns of Biological Evolution," Nature 401 (1999): 881.
36Zhu Shixing and Chen Huineng, "Megascopic Multicellular Organisms."
37Conway Morris, 184.
38Desmond Collins, "The 'Evolution' of Anomalocaris and its Classification in the Arthropod Class Dinocardia (NOV.) and Order Radiodonta (NOV.)," Journal of Paleontology 70, no. 2 (1996): 291.
39T. Peter Crimes, "Trace Fossils and Correlation of Late Precambrian and Early Cambrian Strata," Geology 124 (1987): 107, 108, 109, 110, 111.
40Robert C. Newman, "Progressive Creationism" in J. P. Moreland and John Mark Reynolds, eds., Three Views on Creation and Evolution (Grand Rapids: Zondervan, 1999), 116; and Walter Bradley, "A Response to Howard Van Till," in J. P. Moreland and John Mark Reynolds, eds., Three Views on Creation and Evolution (Grand Rapids: Zondervan, 1999), 219-20, among others, make this claim.
41Conway Morris, 188.
42Raymond C. Moore, Cecil G. Lalicker, and Alfred G. Fischer, Invertebrate Fossils (New York: McGraw-Hill Book Co., Inc.), 224.
43Conway Morris, 192-4.
44Ibid., 192.
45Mikhail A. Fedonkin and Benjamin M. Waggoner, "The Late Precambrian Fossil Kimberella is a Mollusc-like Bilaterian Organism," Nature 388 (1997): 868.
46Rosie Mestel, "Kimberella's Slippers," Earth (Oct. 1997): 29.
47Conway Morris, 160.
48Ibid., 186-7.
49Ibid., 187.
50Ibid.
51Simon Conway-Morris, and J. S. Peel, "Articulated Halkieriids from the Lower Cambrian of North Greenland and their role in Early Protostome Evolution," Philosophical Transactions of the Royal Society of London Bulletin 347 (1995): 332.
52Ibid., 342.
53Fazale R. Rana, "Cambrian Flash," Connections (First Quarter 2000): 3.
54Lazarus J. Salop, Geological Evolution of the Earth During the Precambrian (New York: Springer-Verlag, 1983), v.
55Conway Morris, 162.
56Jere H. Lipps and Philip W. Signor, Origin and Early Evolution of the Metazoa (New York: Plenum Press, 1992), 341.
57Robert L. Carroll, Vertebrate Paleontology and Evolution (San Francisco: W. H. Freeman and Co., 1988), 23.
58Albert Gaudry, Les Enchainements du Monde Animal Dans Les Temps Geologiques, vol. 1, Fossiles primaires, trans. Mark McMenamin (Paris: Libraire F. Savy, 1883), 293-4, cited by Mark A. S. McMenamin, The Garden of Ediacara (New York: Columbia University Press, 1998), 256.
59Stefan Bengtson and Yue Zhao,"Predatorial Borings in Late Precambrian Mineralized Exoskeletons," Science 257 (1992): 367-9.
60Conway Morris, 158-9.
61Stephen J. Culver, "Early Cambrian Foraminifera from West Africa," Science 254 (1991): 689.
62James W. Hagadorn, and Ben Waggoner, "Ediacaran Fossils from the Southwestern Great Basin, United States," Journal of Paleontology 74, no. 2 (2000): 357.
*ASA Member
Glenn Morton has a B.S. in Physics from Oklahoma University and works as a geophysicist in the oil industry. He has been an independent consultant, and served as Manager of Geophysical Training, as Chief Geophysicist for China, and as Geophysical Manager of the U.S. Offshore. He is currently serving as Geophysical Manager of the North Sea. He has published articles on geophysics and published over fifty articles in the area of creation and evolution along with four books, the most recent of which is Adam, Apes and Anthropology.