 Science
in Christian Perspective
Science
in Christian Perspective Science
in Christian Perspective
Science
in Christian Perspective
Dialogue
PALEONTOLOGIC EVIDENCE AND ORGANIC EVOLUTION
The existence and significance of paleontologic evidence, and arguments
for or against the validity of organic evolution.
From: JASA 24 (December 1972): 160-176.
This is the second in a series of Dialogues to be presented in the pages of the Journal ASA. Each published Dialogue is the result of many months of correspondence and feedback between the participants, during which time every effort is mode to eliminate extraneous claims and criticisms.
Like the first Dialogue, this second discussion is also concerned
with that perennial
topic: evolution. Such discussions of evolution may be broken down
into at least
four sub-groups. First there is the discussion of the possibility of evolution
in view of the Scriptural revelation; this was the subject of the
first Dialogue
published in the June 1972 issue of the journal AS\, Second there is
the consideration-and
this is the purpose of this Dialogue-of whether the available evidence indicates
that evolution has taken place. Third is the consideration of how evolution could have occurred. And finally there is
the question
of the compatibility of an acceptance of organic evolution with a
Christian worldview.
Attempts to intermix these four basic questions so as to confuse
their differences
can only result in misunderstanding.
Readers continue to inquire as to why we bother to discuss the
question of evolution,
reliving as it were the days of the Scopes trial in a day far removed
in sympathy
and need. Our answer must be that see exist to serve our readers, and
it is clear
that a sizable minority of our readers consider evolution not only to
be a vexing
problem, hot even one of ultimate and vital concern to their
Christian life. Without
belaboring the subject inappropriately, therefore, we hope that our occasional
excursions into this area will prove beneficial to our readers.
Cuffey's Critique of Moore's Position
Moore's Rebuttal
(Professor John N. Moore is in the Department of Natural Science at
Michigan State
University, East Lansing, Michigan 48823. He is Go-editor of Biology: A Search
for Order in Complexity, Zondervan, Grand Rapids, Michigan 1970 and
Managing Editor
of the Creation Research Society Quarterly.)
Introduction and Definitions
Over 110 years after the publication of Charles Darwin's book, The
Origin of Species
on November 24, 1859, we hear and read, repeatedly, about evolution stated as
fact, in unhesitating fashion, by leading evolutionists. Julian Huxley has said
so in as many words on many occasions and in written form. In 1959,
Fluxicy claimed
even that the universe had evolved, the earth had evolved, life
evolved, man evolved,
and man's culture in sum total had evolved.
In 1966, the now deceased Hermann Muller was instrumental in gaining signatures
of close to 200 prominent scientists in support of the idea that evolution is
as well established as the rotundity of the earth. And Theodosius
Dohzhansky has
said that evolution is as well established as anything could be, according to
all those who are in full possession of the data available.
Little room for credibility seems left for that minority of
scientists (See Olson,
1960), who assert quite boldly that evolution is illogical and not at
all biological.
Nor is some imaginary credibility gap reduced much by someone challenging Gavin
de Beer, who has maintained in print that the certainty of evolution
is comparable
to that of the system of Copernicus, or that of Newton. Yet, I will assert that
evolution is not at all comparable to the systems of either
Copernicus or Newton
with regard to logical precision or probative strength. What can be the basis
of such an allegation?
Actually many, many evolutionists believe that evolution is comparable to the
Newtonian theory in logical precision and probative value, essentially because
they equate evolution with natural selection. Evidently evolutionists
labor under
this impression because they feel as de Beer, i.e.,
Only ignorance, neglect of truth, or prejudice could actuate those who in the present state of knowledge, without discovering new facts in the laboratory and in the field, seek to impugn the scientific evidence for evolution. (de Beer, 1958)
But a close, rigorous check of the de Beer article explicates the fact that he
has equated literally the term "natural selection" with
"evolution",
and then subsequently proceeded to substitute for "natural
selection",
the term "evolution". And de Beer and many, many evolutionists make
the tacit assumption that substantial experimental and field data that may be
used to support the concept of natural selection are also useful as support for
evolution.
Thus I find it necessary to raise questions of logical exegesis with regard to
primary methodological issues associated with evolutionary theory and
interpretations
of several groups of physical data. It would be possible to offer
extensive discourse
around such topics as: a) use and abuse of ad hoc hypotheses, b) ex post facto
explanations, c) the problem of definitions, d) methodological requirements of
genuine scientific hypotheses, e) probability arguments involved in evolution
theory, and f) the problem of untestable hypotheses.
Also I find it necessary to explicate the failure of many, many evolutionists
to recognize overtly the definite limitations of scientific
methodology. As time-binding
organisms, human beings functioning as scientists are still limited
in observational
capacity beyond naked eye study to whatever extensions are possible
through microscopes,
telescopes, ultra-speed films, spectroscopes, and similar instrumentation. And
direct physical data for the historical period of the past may be
studied in archeology
and similar work only some 3,000 years before the present. Thus all discussion
about origin of the universe, the earth, life, man, and man's culture-a la the
previously mentioned statement by Huxley-is pure conjecture.
As background to a discussion of physical evidence and evolution, an
explication
of the meaning of the word "science" or an answer to the
question, "What
is science?", is apropos. Of course the word "science"
comes from
the Latin for knowledge; and, according to a common dictionary
definition, science
is knowledge attained through study or practice. But this definition
is obviously
much too broad to be of much value. For a more coherent definition we find:
Any body of doctrine or collection of truths is scientific to the extent that it yields the power to predict in relation to the subject matter of its choice. ( Somerville, 1941)
And a decade later the following definition was offered:
Science is an interconnected series of concepts and conceptual schemes that have developed as a result of cxperimentation and observation and are fruitful of further experimentation and observation. (Conant, 1951)
And thirdly the Oxford Dictionary contains this formal definition:
A branch of study which is concerned either with a connected body of demonstrated truths or with observed facts systematically classified and more or less colligated by being brought under general laws, and which includes trustworthy methods for the discovery of new truth within its own domain.
Thus, from these three definitions scientific activity involves the search for facts that can be observed or demonstrated, and laws which have been demonstrated also, by means of trustworthy methods of discovery. Then at the core of scientific method or methods is experimental repeatability or reproducibility. Other synonyms for this core idea are predictability and/or control. As a leading paleontologist has pointed out:
The important distinction between science and those other systemizations (i.e., the arts, philosophy, and theology) is that science is self-testing and self-correcting. The testing and correcting are done by means of observations that can be repeated with essentially the same results by normal persons operating by the same methods and with the same approach. (Emphasis added) (Sinspson, 1962)
Therefore, the heart of scientific method is the
problem-hypothesis-test process.
And, necessarily, the scientific method involves predictions. And predictions,
to be useful in scientific methodology must be subject to test empirically. But
is this the case with regard to the theory of evolution? Are
observations involved
that are repeatable?
Thus, many scientists who have critically analyzed the theory of evolution have
found that a General Theory of Evolution must be distinguished from a Special
Theory of Evolution. (See Kerkut, 1960)
A proponent of the General Theory of Evolution,
which is the "Amoeba to Man" thesis, would state that all
living things
in the world have arisen from a single source that came from an
inorganic beginning.
Thus, according to the General Theory of Evolution, the first living
cell "evolved"
into complex muticellular forms of life, these gave rise to all forms
of invertebrates;
in turn, invertebrates "evolved" into vertebrates; fish
into amphihia,
amphibia into reptiles, reptiles into birds and mammals, early
mammals into primates,
and finally primates "evolved" into man. Without question this is the
basic meaning of the term "evolution" for most people.
However, a proponent of the Special Theory of
Evolution would state that many living plants and animals can be observed, over
the course of time, to undergo changes so that new varieties are formed.
Presentation of the General Theory of Evolution as fact has no basis
in science.
The General Theory of Evolution is totally without foundation in
physical evidence
as is shown presently.
But a final word of introduction is needed. I assert that
evolutionists, who speak
and write as "historical" geologists or biologists, do so as men who
present their imagined narratives about the so-called geological
past, and produce
imagined narratives about supposed phylogenetic trees of living
things. Geologists,
especially, must be reminded constantly that they study only the present. Then
they interpret and extrapolate about the past, and in so doing they
leave empirical
science.
Yet, such imagined narratives have been offered for a very long time in geology
textbooks as "accounts" of past "history" of living things.
Such imagined narratives have been presented so persuasively, for such a long
time, that most geologists, paleontologists, and biologists have come to accept
them as fact, as if the events imagined and the supposed changes in living things had occurred actually. Thus, we find Huxley, Muller,
Dobzhansky, and Simpson
in the lead as spokesmen for the position that general evolution is fact.
The Real Situation
What is the real situation? Just what is the situation about general evolution
as fact? The real situation is that discussion about general
evolutionary thought
or theory involves a paradigm case of the "interminable dispute" in
scientific discourse. Discussion about general evolution is plainly a
conceptual
dispute, or a quarrel of faiths. There is no experimentum crucis possible. And
there is no need for new physical evidence as de Beer would have his
readers believe.
There are no private facts for evolutionists; and no private facts
for scientists
who are not disciples of the Evolutionary Faith. Disagreements' are conceptual
in nature, and not factual in character. The same physical data of
the geological
record, animal breeding records, and plant breeding records are used
by both evolutionists
and other scientists.
Also, the real situation could he phrased in terms of "conflict
questions",
as was done in the doctoral thesis, "Methodological Issues in Evolutionary
Theory", by Wing Meng lb for his 1965 degree at Oxford University. Dr. Ho
maintains that these conflict questions are no longer problems of science, but
problems in philosophy. We do not need more physical evidence as per
de Beer for
conflict questions that center in such dichotomies as, 1) mechanism
versus vitalism,
2) mechanistic versus organismic biology, 3) non-teleological versus
teleological
approaches, or 4) non-evolutionary versus evolutionary origin of
matter and life.
Ho sees that empirical versus mm-empirical questions must be faced,
when conflict
questions are formulated. And theories of general evolution involve
conflict questions
about origin that are quite non-empirical. Rather than collection of more facts,
solution or dissolution of conflict questions on origins and general evolution
require analysis and clarification of points at issue according to a particular
viewpoint re meanings, definitions or interpretations'. Resolution of conflict
questions will not come by gaining new physical evidence, but by
making decisions
of intent to construe and apply certain key-terms in some definite manner. Such
key-terms might he listed as,
1. cause, or causes
2. character
3. create
4. development
5. evolution
6. explanation
7. kind
8. life
9. mutation
10, origin
11. prediction
12. probability
13. purpose
14, species
15. succession
16. variation
But, in the main, evolutionists seem unaware of, or uninterested in, precision
of definitions. This seems especially true when evolutionists equate
"evolution"
and "natural selection", or equivocate
"evolution" and "variation".
Or when evolutionary biochemists indiscriminately interchange
"create"
and "synthesize", or "creation and "synthesis". Such
neglect of detail seems contradictory to the spirit of empirical science.
When scientists criticize general evolutionary thought or the use of terms by
evolutionists, when they raise objections to teaching general
evolution as fact, as if it were or is observable, they are merely insisting on
elementary scientific
procedures. The very essence of suspended judgment, as an attitude of
scientists,
and further the self correctiveness of scientific methodology (which
is so often
pointed to as a criterion to separate science from other disciplines of man, as
per Simpson above), are both properly served when scientists ask
pointed conflict
questions above general evolutionary theory or thought.
Scientists, who criticize evolution, experience conflict when they
ask questions
such as, "If a machine is the result of a draftsman and engineer, and if
the draftsman and engineer are the result of their genetic codes, then what is
the organizing principle or pattern for these genetic codes?" If
this question
is pushed back far enough to involve the concept of beginning, or origin, then
solution or dissolution of that conflict question will come only after certain
key-terms are consistently employed by evolutionists.
In sum, then, with regard to the real situation, many scientists maintain that
theories of general evolution are not suitable for the study of origin, whether
concern is for the origin of the universe, the earth, life, man, or
man's culture.
It would seem that something as important to scientists as the origin
of the universe
should not be discussed in basic terms which are employed in a
contradictory manner.
"Evidences" for General Evolution Examined
Therefore, it becomes necessary to examine the broad theory of general organic
evolution, which entails development of an imaginative narrative
about the "history"
of living things, about their origin and changes in the past to the
present. The
thesis of general organic evolution has been well known ever since
Charles Darwin
made it acceptable to the intelligentsia of his time. Specialists and
non-specialists
are acquainted with the evolutionary thesis that all living things
came from organisms
of the past which came from some least complex beginning and in turn
from an inorganic
origin. Thus, change in living things from least complex to most complex is the
"end" involved in general evolution. But the
"means" involved
whereby that "end" supposedly was and is accomplished was imagined by
Darwin to he "natural selection", and evolutionists still hold this
to be a prime mechanism of change.
Darwin used major chapters of his hook to expound upon so-called
"evidences"
for general evolution and the same headings are useful today for reference to
classified physical data as per the following: a) geological record
(succession),
b) morphological affinities, e) geographic distribution, d)
embryological similarities,
and e) rudimentary or vestigial organs. (Blood or protein analyses
would be added
by some today.)
At this point some scientists are quick to point out the practice of
ex post facto
explanations. No one has ever seen one type or form of an animal
change into another
type or form of an animal, and hence all use of physical evidence
under the above
headings partakes of the practice of formulating explanations after the fact.
Darwin and all orthodox disciples of the Evolutionary Faith have
diligently sought
after physical evidence to substantiate the general evolutionary thesis already
expressed simply as "Amoeba to Man", or as one high school textbook
is subtitled: "Molecules to Man". Yet all discussion of
so-called "evidences"
under the above
mentioned headings is done after the fact. Hence the crucial point
still remains
that the basic concepts always involve untestable hypotheses.
And in terms of their methodological approach, scientists are
obligated to point
out that the entire structure of general evolutionary thought rests
upon the geological
record-the supposed historical record of what actually happened.
Yet the whole discussion of supposed succession of horses, or any other type or
form of living thing as based upon the geological record, partakes unavoidably
of the logical fallacy of post hoc ergo propter hoc ("after
this, therefore,
because of it"). The fallacy involves the error of taking something as the
cause for another thing merely because of being earlier in time. That
is, merely
because the remains of one kind of organism lie in a stratum under remains of
another kind of organism, it does not necessarily follow that the
"lower"
is the cause (or ancestor) of the "upper".
Thus some scientists are attempting to construe and apply certain
key-terms with
regard to the geological record. Succession does not afford
sufficient and necessary
grounds for claiming one organism as the ancestor of another.
(Succession in rock
strata is not the same as clear genetic relationship established
through interfertility
tests, which many evolutionists hold as criteria for establishing the species
concept.)
But most important of all is the fact that all of the physical
"evidence"
used by evolutionists under the above headings are made plausible and
persuasive
only because of one basic assumption. Underlying the geological
record, morphological
affinities, geographic distribution, embryological data, rudimentary
organs, and
blood or protein analyses is one basic assumption, i. e., the degree
of relationship
of organisms depends upon the degree of similarity of organisms. In short, if
organisms look alike, then they are related, according to the degree
of similarity.
If organisms do not look alike then they are not related, or only
distantly related,
according to the degree of similarity. But, in no respect, as many scientists
point out, are genetic relationships afforded the general evolutionary thinker
by physical data grouped under the above headings. No genetic relationship is
established through exercise of the assumption that the degree of relationship
depends upon the degree of similarity.
And most conclusively, as far as methodological issues are concerned,
only circumstantial
evidence is involved throughout all the listings of classified
physical evidences
used to support evolution from "Amoeba to Man", or for that matter,
from "Molecules to Man". Relationships expounded are purely
conjectural
because they cannot he tested. All these circumstantial evidences
involve extrapolations
quite beyond the realm of genuine scientific investigation, i. e., experimental
analysis. All hypotheses of relationships of general evolutionary
nature are untestable;
and, therefore, are purely conjectural and speculative. It would
appear, therefore,
that these hypotheses are doomed forever to remain a part of the
untestable dogma
of the Evolutionary Faith.
At this point many scientists would open discussion of the validity
of circumstantial
evidences to the establishment of scientific truth. Being reminded
that we cannot
equate "natural selection" to "evolution", and we
cannot equivocate
"evolution" with "variation", critical scientists
press hard
on the fact that general evolutionary theorists, in using
circumstantial evidences
almost ex
elusively, are involved with an important weakness and seriously irremediable
defect in their thinking. This is their heavy dependence on the argument from
analogy. An analogy can he given:
If (A) is known to have properties "P" and some additional property "R" and resembles (A), in that (A) is
known to have properties "P", then (A) is expected to have property "R".
Darwin depended on an analogy between artificial
selection and natural selection, as he discussed his supposed
mechanism for general
evolution. He formulated the reasoning that the artificial selection
of the breeder
and fancier of domestic animals, about which he could observe and gain actual
physical data, was analogous to his imagined natural selection of the
better adapted
organisms for survival. But the analogy breaks down.
In the first place, artificial (breeder) selection must be
accomplished in accordance
with certain desired or determined criteria. The plant breeder has
distinct characteristics
which he wants to retain, improve, or even remove, if possible, for
his particular
desire (criteria). The breeder works with plants to bring about
distinct departures
in characters according to this design. This also is true of the animal breeder
or fancier.
In the second place, proponents of the doctrine of
natural selection state that it occurs without any set
criteria. There are no distinct characteristic changes planned or
designed. Only
the interaction of organism (s) (populations) and the environment are involved.
Plants change according to wind pollination or as insect pollination
occurs. Animals
reproduce and control a territory and change according to interaction with the
environment, somehow. There are no criteria. Furthermore, supposed changes are
slight, minute, hardly noticeable variations of the genome. Actually most
distinct
departures (most mutants) are eliminated, and field and laboratory
data are better
interpreted that gene stability is the most proper conclusion from
empirical data.
Artificial selection, therefore, is not analogous to natural selection, or vice
versa. There is no resemblance between A and A' because the
properties associated
with A are different from the properties associated with A. Thus, there is no
adequate comparison of artificial selection and supposed natural selection and
the analogy fails.
Genetics as "Evidence" for General Evolution
As a last defense for general evolution, many will demand,
"Well, what about
genetics? Aren't evolutionists on the correct path when they use data
from genetics
to try to support their thesis of 'amoeba to man' evolution? Is it
not true that
variations have been shown to be transmissible?" Yes, "Is it not true
that changes of genetic material have been shown to be of a fixed nature?"
Yes. "Is it not true that changes of genetic material are
constantly arising?"
Yes.
But many scientists are asking, "Is there any evidence
of empirical nature that favorable variations have ac
cumulated so as to effect overt general evolutionary changes?"
Again, a conflict
question has been reached, and the problem of defining the meaning of
terms must
be faced. "What is a viable mutation?" "What is a
variation?"
"What is an evolutionary change?"
Clearly, even evolutionists must admit that no new organs or organisms, re type
or form, have come about by the shuffling and reshuffling of genes. It is true
that
the researcher may conclude from his experimental data that changes
in eye color,
in eye shape, in eye pattern in fruit flies do occur, but the eyes
always remain
Drosplnlia eyes, if that is the organism with which he deals in his research!
Recombinations of genetic materials do not bring about new types or forms. Such
changes are always within limits of known types or forms of organisms.
That inviolate genetic barriers exist between major groups of living things may
be stated conclusively on the basis of available genetic evidence. Unbridgeable
breeding gaps are known; no amount of reference to ploidy and or
chromosomal rearrangements
will truly erase the undeniable evidence that breeding gaps between
major groups
of living things do in point of fact actually exist.
Anyway any reference to different phenomena of ploidy and chromosomal
rearrangements
constitutes nothing more than ad hoc, untestable hypothesizing, as far as any
attempt to explain any relationships between or among major groups of animals
or major groups of plants is concerned. Absolutely no genetic connections are
ever established between major groups of living things by means of
any mechanisms
involving ploidy and chromosomal rearrangements.
But there is another problem here. Are mutations,
or more properly mutants, truly raw materials upon which
"natural selection"
operates, as is so commonly claimed by such as Theodosius Dobzhansky? lie has
admitted that mutants do not of themselves involve anything new
(Dobzhansky, 1953).
Mutations are sources only of differences of characteristic
expressions of traits
already in existence, and not a source of new traits. Mutations result only in
changes within the existing genetic structure. Therefore the
fundamental genotype
remains unchanged as far as traits are concerned.
Thus the contention so often heard and read that mutations supply the
raw materials
for "natural selection" to bring about "amoeba to
man" evolution
involves a whole hierarchy of ad hoc hypotheses, which are void of testability.
Once again the untestable hypothesis is encountered, which is so
common in general
evolutionary theory or thought.
Since the vast majority of mutations are lethal or cause impairment of physiology
of the organism, since the gene mutation hypothesis suffers from the
difficulties
of the pathological nature of and the great rarity of mutational
changes, it follows
that mutations are not useful as supporting evidence for general
evolution, that
is, "molecules to man". And public attestations to the
"failure"
of the mutational theory are appearing in print more and more. As one scientist
has written: "But who can tell us how point mutations and sundry
tape doublings,
crossings, and writhings made the oak and squirrel, the gull and the
gall by summing
up the changes in many a piece of enzymes?" (Morrison, 1971 and
Davis, 1970;
Haskins, 1971)
Any hypotheses about "suppressor" genes (Fisher, 1932),
undetected viable
mutations (East, 1936), or changes in the evirmment favoring certain mutations
(Dobzhaosky, 1953) must be labelled untestable. And a similar
generalization can
be made of more recent attempts to "explain" change of one
kind of organism
into another kind of organism by way of mutations and other gene
manipulations.
Thus an important methodological issue with regard to physical
evidence from genetics
is the fact that the
favorite hypotheses of evolutionists fail to satisfy the criterion of
testability,
and because of this., they lie outride the realm of scientific investigation.
In genetics, many scientists detect the repeated practice of ad hoc hypotheses,
which are fully untestable, and detect heavy commitment by general
evolutionists
to extrapolation and interpretation of terms that are vague and
ambiguous. "What
is a viable mutation?" "What is a useful mutation?"
In considering for a moment that last question, a change of color in moths or
alteration of food use by bacteria might be cited as results of
"favorable"
or "useful" gene mutations. Nevertheless such changes of
moths or bacteria
arc only within a certain genus, and not across limits of genera.
Therefore, any
thought to consider any so-called "favorable" gene
mutations as possible
mechanisms for changes across limits of known kinds, which are the
type of changes
required if the general theory of evolution is to he given any
empirically sound
basis, partakes again of dependence upon ad hoc, untestable hypotheses.
In summation, with regard to physical evidence from genetics, the
point that needs
to be emphasized over and over again is that minor changes can and do occur in
living organisms, but the changes are always \vithin bounds of a certain type,
form, or kind. And in passing, it should be noted that even in the
fossil record,
basic types, forms, and kinds are clearly recognizable even as we see
them today
in many, many examples.
Of course, this is in exact agreement with the pattern found in Genesis 1, that
is, "after their kind", "after his kind". This
can be extended
by the statement that all the known physical evidences can be fitted into the
Genesis account in great consistency with all the better scholarship; and this
cars he done better by far than attempts to fit the physical evidence
into imagined,
speculative narratives of evolutionary theorists.
On the basis of the most rigorous scholarship, the conclusion is
inescapable that
no transitional forms of true genetic relationship or connection can
be established
from breeding records, which constitute the only truly repeatable, demonstrable
physical evidence (hence really scientific). There is truly an irrefutable case
that can he made for "fixity of kinds".
Conclusion
Because of failure to follow fundamental scientific procedures, especially with
regard to origins, because of the extensive commitment of general evolutionists
to sheer circumstantial evidences, because of the failure of
mutational hypotheses
to provide anything pertaining to truly new physical traits, it is clear that
theories of general evolution are not suitable for the study of
origins, whether
concern centers on origin of the universe, the earth, life, man, or
man's culture.
And equally important, theories of general evolution cannot be
presented as fact
without implication in fraud and/or hoax.
REFERENCES CITED
Conant, James B. 1951 Science and common sense, New Haven: Yale
University Press,
p. 25.
Davis, Bernard. 1970 Prospects for genetic intervention in
man, Science, 170, 18 December: 1279-1283.
de Beer, Gavin. 1958 The Darwin-Wallace centenary, Endeavor,
April, p. 75.
Dobzhansky, Theodosius. 1953 Genetics and the origin of species. New
York: Columbia
University Press, p. 296.
East, E.M. 1930 Genetic aspects of certain problems in evolution,
American Naturalist,
Vol. 70.
Fisher, Ronald. 1932 The genetic theory of selection. Oxford: Oxford University
Press.
Haskins, Caryl. 1971 Advances and challenges in science in
1970, American Scientist, 59, May-June: 298-307. (See especially
"Molecules
and evolution" section: 304-306.)
Ho, Wing Meng. 1965
Methodological Issues
in Evolutionary
Theory with Special Reference to Darwinism and Lamarekism. Oxford:
Bodleian Library
Oxford. (Shelfmark: Ms. D. Phil. d. 3591. Photographic order no. BPC
7442, Oxford
University Press.)
Kerkut, CA. 1960 Implications of evolution. New York:
Pergamon Press.
Morrison, Philip. 1971 Book Review, Scientific American, 224
(5), May: 128.
Olson, E.C. 1960 (in) Evolution alter Darwin, Vol. 1. Edited
by Sol Tax. Chicago: University of Chicago Press, p. 523.
Simpson,
CC. 1961, 1962
Notes on the nature of science by
a biologist (in) Simpson, CC. and Others (Editors) Notes on the
nature of science.
New York: Harcourt. Brace and World, Inc., p. 9.
Sommerville, John.
1941 Umbrellaology,
or methodology in
social science, Philosophy of Science, Vol. 8: 560.
Cuffey's Critique of Moore's Position
The critical role of paleontologic evidence in demonstrating organic evolution
to the satisfaction of the scientific community seems largely
overlooked by writers
of Moore's persuasion. Consequently, presenting such evidence here in
non-technical
fashion seems to me to he the most useful contribution which these papers can
make toward resolving the evolution controversy.
Moreover, the arguments used against this paleontologic evidence by
anti-evolutionists
like Moore are woefully lacking, because they rest upon
misunderstanding or oversimplification
of actual paleontologic procedures. Four brief comments suffice to
elaborate this
point.
First, as an example, Moore's suggestion that the stratigraphic succession of
fossils is logically fallacious is hated upon a grossly and
erroneously oversimplified
view of the nature of the fossil record. As explained previously in my position
paper, it is important not only that one organism's remains lie below those of
another. It is also essential, for demonstration of evolutionary relationship
between the two, that the intervening strata contain other fossils which grade
continuously in both morphology and ehronologic-stratigraphic position from the
lower to the upper form.
Similarly, as a second example, the curious notion that studying past
events involves
only speculation and untestable hypotheses reflects serious ignorance. Actual
paleontologic practice is in fact dominated by observational investigation of
the fossil materials which would have been produced under various
possible circumstances,
in an attempt to determine how nature most probably did behave in the past.
Third, as previously indicated, the paleontologic record provides an
immense and
overwhelming quantity of evidence supporting evolutionary concepts. In general,
retreat into oversimplified philosophical arguments against such a massive body
of verifiable observational evidence suggests strongly an inability
to convincingly
counter the clear implications of that evidence.
Fourth, Moore states that disagreements concerning evolution are "quarrels
of faiths". In contrast, as indicated earlier, I believe that
such disagreements
are readily resolvable by scientific data. I sincerely hope that those of his
persuasion will reject one possible implication of his statement-namely, that
no matter what relevant evidence is newly presented to them, they will not consider the implications of that evidence! Retaining open minds
about controversial concepts is necessary, until sufficient evidence
accumulates.
However, enough scientific evidence is already at hand to remove any reasonable
doubt about the validity of the concept of organic evolution.
Other points raised by Moore are adequately covered in my position paper, and
therefore need not he repeated here.
In rebuttal to Cuffey's critique, I assert that I am quite aware of "the
critical role of paleontologic evidence" with regard to supposed organic
evolution. It is my concern about misuses of such information that prompts me
to point out again that no demonstration empirically of general evolution has
been accomplished. To allude to the "satisfaction of the
scientific community"
seems to me to be no more than an appeal to the fallacious idea that truth is
a matter of voting.
The "scientific community" was satisfied with the
Copernican formulations;
and yet, Kepler wrought great and significant changes. The scientific majority
was satisfied with Newtonian physics; and yet, Einstein wrought great
and significant
changes. Contemporary scientists of Charles Darwin were at one moment satisfied
with their interpretations of Genesis 1; and yet, Darwin wrought
great and significant
changes.
It is just because of my understanding and appreciation of the
complexity of actual
paleontological procedures that I make bold to tell it like it is,
and urge fellow
colleagues in the scientific community today to realize, that now is the time
for all scientists to reconsider general evolution. A period of over 110 years,
since Darwin's book appeared, is time enough to insist that
evolutionists either
put up hard physical evidence for general evolution, or else yield in
their arrogant
dogmatism in writing and teaching about general evolution as fact. To challenge
scientists in astronomy, biochemistry, botany, embryology, geology,
paleontology,
and zoology to provide hard physical evidence is done in the spirit
of self-correctiveness
of scientific endeavor mentioned in the Simpson quote in the Introduction of my
position paper.
And Cuffey's use again of such words as "demonstration",
"observational",
and "implications" in his critique must be challenged. He
did not write
of, and he cannot provide, any empirical demonstration of genetic
lineage between
or across limits of kinds of organism. He joins his reference to
"observational"
with "possible" and "probable" and thus provides
further basis
for my case that he does deal inescapably in "speculation and untestable
hypotheses". And when he asks that critics of evolution consider
the implications
of physical evidence, I offer that I have done just that per my position paper,
and I repeat that the real situation that prevails is total absence
of any physical
evidence upon which to base the General Theory of Evolution. Any discussion of
change of species or genetic variation within limits of kinds of organisms must
never be confused with general evolution.
To speak of "validity", as Cuffey does in his next to
closing statement
of his critique, leads directly to the whole thrust of my criticisms
of any presentation
of general evolution as fact.
There is immense "reasonable doubt" about the
validity of general evolution. There is immense "reasonable
doubt" that
general evolution has ever occurred. All of the physical data from comparative
anatomy, comparative embryology, rudimentary (vestigial) organs,
blood and protein
analyses, Mendelian and population genetics, and the fossil record
may be fitted
more validly into the creation account of Genesis 1, than into any speculative,
imaginative narrative of men about general evolution.
I hope sincerely that those of Cuffey's persuasion will reject one
possible implication
of his statement before concluding his position paper, that
Christians "will
need to integrate evolutionary process into their views as being the proximate
means which Cod uses to create various forms of life"-namely,
the implication
that the ways of men, the ideas of men, the
traditions of the world must be given credence over the ways of
Christ, who said,
"male and female created he them". If Christians accept the ideas of
men about general evolution, then they may be consciously or
unconsciously beguiled
(Cal. 2:8 and Eph. 4:14) to accept a human substitute about origins
for the Word
of God, which is the one and only source of unchanging answers for
people of all
generations about origins of the universe, the earth, life, man, and
man's culture.
Today, Christians can declare confidently that "fixity of kinds" is
the scientifically documented prediction from the creation model,
that is, supported
by all physical evidence. And "fixity of kinds" might well
be understood
as the modern day equivalent of the Biblical "after his
kind" or "after
their kind".
THE POSITION OF ROGER J. CUFFEY
Moore's Critique of Cuffey's Position
(Professor Roger I. Cuffey is active in the field of paleontology,
and is in the
Department of Geosciences, The Pennsylvania State University, University Park,
Pennsylvania 16802.)
Introduction
Practicing paleontologists today, regardless of personal philosophical outlook,
unanimously agree that the varied organisms inhabiting the earth originated by
a process of gradual, continuous development or evolution over long periods of
prehistoric time. Because the case for organic evolution had been
adequately demonstrated
in the late 1800's (principally by paleontologic evidence), scientists in this
century turned their attention to many other important subjects. Consequently,
most have been surprised by (Lewontin, 1971) and also illprepared to cope with
the recent reappearance of anti-evolutionary ideas (such as Morris,
1963; Moore,
1970a, 1970h, 1971a, 1971h; Moore & Slusher, 1971). Therefore, presenting
the paleontologic evidence relevant to the concept of evolution is most timely,
particularly for an audience like that of the Journal ASA.
The participants in the current controversy about evolution seemingly
agree that
fossils (the study of which comprises the science of paleontology)
are the remains
(or direct traces) of formerly living organisms, preserved in the earth's crust
since prehistoric times. This conclusion is incontrovertibly supported by the
complete spectrum observable within the earth's crust between
recently dead organisms
and highly altered fossils.
In addition to the morphology of fossils, a paleontologist studies also various
aspects of their distribution within the earth's crust, As Van de Fliert (1969)
has ably discussed, the rock layers comprising that crust reveal a
chronological
framework (usually stated succinctly as the standard geological time scale) for
the earth's history. This basic framework, founded upon repeatable observations
of the succession of rock strata, is quite independent of any concept
of organic
evolution (Van de Fliert, 1969, p. 75, 77); in fact, the standard
time scale historically
was worked out half a century before evolution was proposed and
demonstrated.
Fossil Sequences
As a consequence, we can examine the fossils entombed in
chronologically successive
rock layers, and thereby learn what organisms inhabited this planet
during successive
intervals of past geologic time. When we do this, we find that the
fossils naturally
form sequences showing gradual and continuous morphologic changes from earlier
forms to later forms of life, sequences which make evolutionary interpretations
ultimately inescapable.
As working paleontologists interested in the history of particular organisms,
we locate for detailed study a relatively thick succession of
fossil-bearing rock
layers whose observable physical features indicate continuous and uninterrupted
deposition over a comparatively long time interval. We next examine
those layers
for
the fossils in which we are interested. We initially find a few
fossils, scattered
widely among the different layers. Studying these specimens usually
shows noticeable
morphological differences between ones from various geologic ages, differences
which we recognize formally in progress reports by referring the specimens to
different species, genera, etc., depending upon the magnitude of
those differences.
Continued field collecting from the rock strata intervening between
any two successive
forms thus described frequently produces a series of fossils which begin with
the earlier form, change in morphology gradually and continuously as we proceed
upward, and end up with the later form. Because these new fossils demonstrate
a morphological and parallel chronological transition from the earlier form to
the later form, they are termed "transitional fossils".
Examples of Transitional Fossils
If we read the paleontologic literature (especially if with the background of
professional paleootologic training and experience; Cuffey, 1970, p.
93), we find
that the fossil record contains many examples of such transitional
fossils. These
connect both low-rank taxa (like different species) and high-rank
taxa (like different
classes), in spite of the record's imperfections and in spite of the relatively
small total number of practicing paleontologists. Because of the critical role
which transitional fossils played in convincing scientists of the occurrence of
organic evolution, paleontologists have been appalled that many
otherwise wellinformed
persons have repeated the grossly misinformed assertion that
transitional fossils
do not exist. Consequently, after a relatively brief and non-exhaustive search
of the literature immediately available to me, I compiled the
examples of transitional
fossils presented here. At least enough of these can be readily
examined by anyone
seriously interested in this topic that he can be convinced of their
implications,
I believe; collectively, they (and the many other similar ones which
more extended
search would find) comprise a massive body of evidence which cannot he ignored
or explained away.
Although the broad patterns and many details in the history of life
are well known,
many other details remain to be learned. Because of the unevenness of
our knowledge,
therefore, we can conveniently distinguish several different types of
transitional-fossil
situations. Let us consider these now, starting with that situation where our
knowledge is most complete, and proceeding through situations in
which knowledge
is progressively less complete.
First, some groups have been so thoroughly studied that we know
sequences of transitional
fossils which grade continuously from one species to another without break (Table 1), sometimes linking several successive species which cross
from one higher taxon into another (Table 2). We can say that
situations of this
kind display transitional individuals. Among the many available
examples of transitional
individuals, some particularly convincing examples can be noted.
These involve:
carols (Carruthers, 1910, p. 529, 538; Easton, 1960, p. 175; Moore, Lalicker, & Fischer, 1952, p. 140; Weller, 1969, p. 123),
gastropods (Fisher, Rodda, & Dietrieh, 1964),
peleczjpods (Kauffman, 1967; Kauffman, 1969, p. N198-200; Kauffman, 1970, p. 633),
echinoids (Beerbower, 1968, p. 136, 138; Kermack, 1954; Nichols, 19S9a, 1959h; Olson, 1965, p. 98; Rowe, 1899).
Second, other fossil groups have been well enough studied that we know sequences of transitional fossils comprising a series of chronologically successive species grading from an early form to a later form (Table 3), again sometimes crossing boundaries separating different higher taxa (Table 4). This type of situation can be termed successive species. Published descriptions of successive species lack explicit discussion of individuals transitional between the species, although frequently such exist in the author's collection but are not discussed because they are not directly pertinent to his purposes. Again, some especially persuasive examples of successive species can he seen, among:
forominiferons (Wilde, 1971, p. 376),
brachiopods (Greiner, 1957; Raup & Stanley, 1971, p. 124),
pelecppods (llastoo, 1960, p. 348; Kay & Colbert, 1965, p. 327; Moore, Lalicker, & Fischer, 1952, p. 447; Newell, 1942, p. 21, 42, 47-48, 51-52, 60, 63, 65; Olson, 1965, p. 97; Stenzel, 1949; Stenzel, 1971, p. N1079-1080; Weller, 1969, p. 209),
ammouoids (Cobhan, 1961, is. 740-741).
In many fossil groups, our understanding is relatively less complete,
thus giving
rise to a third type of situation which we can label successive
higher taxa. Here,
we may not have complete series of transitional individuals or
successive species,
but the genera (or other higher taxa) represented in our collections
form a continuous
series grading from an earlier to a later form, sometimes crossing from one higher-rank taxon into another
(Table 5). Because
genera are relatively restricted in scope, many series of successive
genera have
been published. However, families and higherrank higher taxa are so
broad in concept
that they are not usually used to construct transitional-fossil
sequences, although
occasionally they are (Bulman, 1970, p. V103-104; Easton, 1960, p. 436; Flower
& Kummel, 1950, p. 607).
Finally, in some fossil groups, our knowledge is quite fragmentary and sparse.
We then may know of particular fossils which are strikingly intermediate
|
Table 1. Examples of transitional individuals grading continuously
between successive
species within the same higher taxon (genus). Algae: Gartner, 1971. Angiosperms: Chandler, 1923, p. 124, 132-133; Chancy, 1949, p. 197-198; Stebbins, 1949, p. 230-231. Forominiferans: Barnard, 1963, p. 82, 90; BauzerChcmousova, 1963, p. 48. Corals: Carruthers, 1910, p. 529, 538; Cocke, 1970, o 13 Raston, 1960, p. 175; Moore, Lalicker, & Fischer, 1952, p. 140; Ross & Ross, 1962, p. 1182-1184; Weller, 1969, p. 123. Bryozoans: Coffey, 1967, p. 38-39; Coffey, 1971a, p. 158; Coffey, 1971b, p. 38; Elias, 1937, p. 311, 317. Brachiopods: Ziegler, 1966, p. 532. Gastropods: Fisher, Rodda, & Dietrieh, 1964; Lull, 1940, p. 19; SohI, 1967, p. B12-13, B1516; Thomson, 1925, p. 96. Pelecypods: Charles, 1949; Charles & Maubeuge, 1952, 1953a, 1953h; Heaslip, 1968, p. 58, 69, 77-79; Imlay, 1959; Kauffman, 1965, p. 8-21; Kauffman, 1967; Kauffman, 1969, p. N198-200; Kauffman, 1970, p. 633; Kay & Colbert, 1965, p. 325; Lerman, 1965, p. 416, 431-432; MacNeil, 1965, p. G35-36, C42; Raop & Stanley, 1971, p. 191, 257; Stenrel, 1971, p. N1077; WaIler, 1969, p. 26. Ammanaids: Cohhao, 1958, p. 114; Cobban, 1962a, 1962h; Cobban, 1969, p. 6; Cahban & Reeside, 1952, p. 1020-1022; Easton, 1960, p. 456. Trilobites: Broower, 1967, p. 152-155; Kaufmano, 1933, 1935; Raop & Stanley, 1971, p. 292; Simpson, 1953, p. 250. Echiooids: Beerbower, 1968, p. 136, 138; Durham, 1971, p. 1126-1127; Hall, 1962; Kermaek, 1954; Nichols, 1959a, 1959b; Olson, 1965, p. 98; Rowe, 1899. Conodonts: Clark, 1968, p. 21-23; Scott & Callinsan, 1959, p. 562. Marnosols: Osborn, 1929, p. 20-21; Simpson, 1953, p. 387-388; Teilbard de Chardin, 1950; Trevisan, 1949; Watson, 1949, p. 47; Wood, 1949, p. 188-189. |
|
Table 2. Examples of transitional individuals grading continuously
between successive
species and crossing from one higher taxon into another. Cinkgophytes; Andrews, 1961, p. 337-339; Brown, 1943, p. 863; Franz, 1943, p. 323; Scagel et at, 1965,1). 484; Seward, 1938; Weller, 1969, P. 66. Angiosperms: Chancy, 1949, p. 193-199; Elias, 1942, p. 70-71, 88-89, 109-122; Stehhins, 1949, p. 230. Foramiferans: Banner & Blow, 1959, p. 21; Barnard, 1963, p. 86, 88-89; Gimbrede, 1962, p. 1112-1123; Jones, 1956, 1). 274; Papp, 1963, p. 352353 Woodland, 1958, p. 803-808; Zeller, 1950, p. 19. Brachiopods: Boocot & Ehiers, 1963, p. 48-51. Pelecypods: Newell, 1942, p. 21, 59. Anononoids: Arkell, Kommel, & Wright, 1957, p. L113119; Briokmann, 1929, 1937; Broower, 1967, P. 156-158; Cobban, 1951, p. 5-11; Cobhan, 1964, p. 110-14; Easton, 1960, p. 455; Erben, 1966; Knimbein & Sloss, 1963, p. 369; Olson, 1965, P. 105-107; Raop & Stanley, 1971, p. 264, 306-307; Spath, 1938; Wenger, 1957. Conodonts: Rexroad, 1958, p. 1158. Mammals: Hanson, 1961,p. 50-51; Scott, 1937, p. 417; Simpson, 1951, p. 114-121, 148, 217-228, 232, 236, 257, 265, 282, p1s. 20, 31; Wood, 1949, P. 186. Honsinida: Coon, 1962; Howells, 1967; Kommcl, 1970, p. 578-583; Le Cros Clark, 1964; Uzzell & Pilbeam, 1971, p. 615. |
between two relatively high-rank higher taxa, but which are not yet connected
to either by a more continuous series of successive species or
transitional individuals.
We can refer to these as isolated intermediates, a fourth type of
situation involving
transitional fossils, a type which represents our least-complete
state of knowledge.
Isolated intermediates include some of the most famous and
spectacular transitional
fossils known, such as Archaeopteryx (Colbert, 1969, p. 186-189; Romer, 1966,
p. 166-167). This form is almost exactly intermediate between the
classes Reptilia
and Ayes (Cuffey, 1971a, p. 159; Cuffey, 1972, p. 36), so much so
that "the
question of whether Archaeopteryx is a bird or a reptile is unimportant. Both
viewpoints can be defended with equal justification" (Brouwes,
1967, p. 161).
The fossil onychophorans (Moore, 1959, p. 019; Olson, 1965, p. 190)
and the fossil
monoplacophorans (Knight & Yochelson, 1960, p. 177-83; Raup & Stanley,
1971, p. 308-309) have been regarded as annelidarthropod and
annelid-mollusk inter-phylum
intcrsnediates, respectively. Moreover, although invertebrate phylum
origins tend
to be obscure for several reasons (Olson, 1965, p. 209-211), recently
discovered,
Late Precambrian, soft-bodied invertebrate fossils may well alter
that situation,
particularly after certain peculiar forms are studied and compared with Early
Cambrian forms (Kay & Colbert, 1965, p. 99, 103; Weller, 1969, p. 247).
Mention of this last prompts me to point out parenthetically that the
appearance
of shelled invertebrates at the beginning of the Cambrian has been
widely misunderstood.
The assertion is frequently made that all the major types of animals appeared
suddenly and in abundance then. In actual fact, collecting in successive strata
representing continuous sedimentation from Late Precambrian into Early Cambrian
time reveals a progressive increase upward in abundance of
individuals. Moreover,
the various higher taxa-particularly the various classes and orders reflecting
adaptation to different modes of life-appear at different times spread over the long
interval between the Early Cambrian and the Middle Ordovician.
Finally, because of widespread interest in questions of man's
origins, it is well
worth emphasizing that a rather complete series of transitional fossils links
modern man continuously and gradationally hack to midCenozoic,
generalized pongids
(see references in Table 2).
In spite of statements to the contrary . . . , the fossil record of the Hominnidea, the superfamily containing
man and the apes, is quite well known, and it is therefore possible to outline a tentative evolutionary scheme for this group (Uzrcll & Pilbeam, 1971, p. 615).
Potential Complications of the Paleontologic Literature
Non-paleontologist readers examining examples of transitional fossils mentioned
above should be aware of several common occurrences within the
professional palcontologic
literature which could conceivably he confusing.
Historically, continued paleontologic research on any particular fossil group
tends to move our understanding of its fossil record from the least-complete to
the most-complete type of transitional-fossil situation. For example,
early paleontologists
recognized that the goniatite ammonoids gave rise to the ceratite
ammonoids (successive
higher taxa, in this case superorders or infraclasses; Easton, 1960, p. 436);
later work indicated the successive species by which this transition
was accomplished
(Easton, 1960, p. 446; Miller, Furnish, & Schhsdewolf, 1957, p. L22). Other
examples can also he cited (Simpson, 1953, p. 361-364; Cuff cy, 1967,
p. 38-39).
Also, our ideas about particular lineages may sometimes change as
more specimens
are brought to light (Stenzel, 1971, p. N1068-1070, 1077).
Frequently, secondary references portray evolutionary lineages much more vividly than does the
|
Table 3. Examples of successive species within the same higher taccon
(genus). Angiosperms: Chandler, 1923; Chancy, 1949, p. 197199; Elias, 1942; Stehbios, 1949, P. 230-231. Foraminiferans: Barnard, 1963, p. 82; Bronnimann, 1950, p. 406; Cita-Sironi, 1963, p. 119-121 Hottinger, 1963, p. 306-307; Schanh, 1963, p. 288290, 292-294; Wilde, 1971, p. 376. Brachiopods: Berry & Boneot, 1970, p. 30-31; Dunbar & Waage, 1969, p. 113; Greiner, 1957; Ranp & Stanley, 1971, p. 124. Gastropods: Franz, 1932; Franz, 1943, p. 272; SohI, 1960, p. 100. Pelecypods: Deehaseaux, 1934; Easton, 1960, p. 348; Heaslip, 1968, p. 74-77, 79-81; Kay & Coibert, 1965, P. 327; Lerman, 1965, p. 416; Moore, Lalieker, & Fischer, 1952, p. 447; Newell, 1937, p. 40, 80; Newell, 1942, p. 21, 42, 47-48, 51-52, 60, 63, 65; Olson, 1965, p. 97; Sehafle, 1929, p. 79; Steorel, 1949; Stenrel, 1971, p. N1056-1057, N1077, N1079-1080; Weller, 1969, p. 209; Zeuner, 1933, p. 317. Trilobites: Grant, 1962, p. 983-998. Crustaceans: Guber, 1971, p. 15-16; Soho, 1962, p. 1207; Swartz, 1945; Weller, 1969, p. 267. Garpoida: Barrande, 1887; Weller, 1969, p. 297. Blastoids: Beaver, 1967, p. S303-305. Craptolitea: Berry, 1960, p. 9. Fishes; Boreske, 1972, p. 3-4. Amphibians: Olson, 1965, p. 45-48. Mammals: Lull, 1940, p. 189; McGraw, 1937, p. 448; Tedford, 1970, p. 671, 694. |
original paper reporting them. For instance, contrast the original presentation
of one coral sequence (Carruthers, 1910, p. 529, 538) with several
later presentations
(Easton, 1960, p. 175; Moore, Lalicker, & Fischer, 1952, p. 140; Weller,
1969, p. 123).
Sequences of transitional individuals or successive species are
often, especially
for teaching purposes, presented instead as more generalized
sequences of successive
genera. One ammonite lineage including transitional individuals
between families
(Spath, 1938; Arkell, Kummel, and Wright, 1957, p. L113-116) appears elsewhere
as merely successive genera (Olson, 1965, p. 105-107). The various successive
species of the horse lineages (Simpson, 1951, p. 114121, 217-228,
282) are often
summarized as successive genera (Hanson, 1961, p. 50-51; Scott, 1937,
p. 417).
Similarly, for instructional purposes, some authors illustrate a
series of fossils
which show a progression in morphology, but which are not
chronogically successive.
These therefore are not evolutionary sequences, even though they resemble such.
Two examples of such morphological series involve foraminiferans (Pokorny, 1963,
p. 312) and nautiloids (Easton, 1960, p. 426).
In many instances, transitional individuals exist but are not
reported explicitly
as evolutionary lineages, for several reasons. Fully documenting such complete
sequences is rather expensive in both research effort and publication
cost; thus,
many remain unpublished (Berry & Boucot, 1970, p. 30-31).
Moreover, the practicing
paleontologist sees little need to repeatedly reprove
well-established concepts,
especially when his primary concern is with other matters such as
biostratigrapluc
dating (Berry, 1960, p. 9).
Effect of Transitional Fossils on Taxonomic Practises
Still further, because the Linnean system of taxonomic nomenclature
has been very
useful historically, we tend to refer transitional individuals to that species
which they resemble most, rather than calling attention
nnmenelaturally to their
intermediate status (Bird,
|
Table 4. Examples of successive species crossing from one higher
taxon into another. Ginkgophytes: Andrews, 1961, p. 337-339; Brown, 1943, p. 863; Franz, 1943, p. 323; Scagel et al, 1965, p. 484; Seward, 1938; Weller, 1969, p. 66. Foraminiferans: Berggren, 1962, p. 101, 116-126. Bryozoans: Lang, 1921-1922; Easton, 1960, p. 268. Gastropods: Fisher, Hodda, & Dietrieli, 1964. Pelecypods: Stenzel, 1971, p. N1057, 1078. Nautiloids: Easton, 1960, p. 425; Flower, 1941, p. 526; Moore, Lalicker, & Fischer, 1952, p. 351. Annnonoids: Arkell, Kumnsel, & Wright, 1957, p. L116; Cohhan, 1961, p. 740-741; Easton, 1960, p. 446; House, 1970, p. 666-674; Miller, Furnish, & Sehiodewoif, 1957, p. L22; Wright & Wright, 1949. Crustaceans: Claessner, 1960, p. 40-41; Glaessner, 1969, 1). R410-411. Grinoids: Moore, Lalieker, & Fischer, 1952, p. 629. Echinoids: Jackson, 1912, p. 231; Weller, 1969, p. 355. Reptiles: Lull, 1940, p. 290; Olson, 1965, p. 99-101. Reptile-Mammal Transition: Olson, 1965, p. 202. Mammals: Kummel, 1970, p.514; Lull, 1940, p. 524; Matthew, 1910; Nelson & Seniken, 1970, p. 3734; Osborn, 1929, p. 35-37, 724, 761, 773, 784, 791, 801, p1. 48; Patterson, 1949, p. 243244, 246, 263, 268; Scott, 1937, p. 429; Simpson, 1951, p. 148, 245; Wood, 1949, p. 188-189. |
|
Table 5. Examples of successive higher taxa (genera). Coniferophptes: Florin, 1951; Seagel et al, 1965, p. 491-492, 520-522, 596-597. Foraminiferans: Dunhar, 1963, p. 42; Pokorny, 1963, p. 155, 192. Corals: Wells, 1956, p. F364. Brachiopods: Dunhar & Rodgers, 1957, p. 280; Slsroek & Twenhofel, 1953, p. 346. Nautiloids: Teiehert, l964a, p. K200-201 Teichert, 1964h, p. K325. Amaionoids: Miller, Furnish, & Sehindewolf, 1957, p. L23. Colceids: Easton, 1960, p. 476; Weller, 1969, p. 233. Blastoids: Fay, 1967, p. S394-395; Tappan, 1971, p. 1087. Crinoids: Moore, Lalicker, & Fischer, 1952, p. 631. Echinoids: Kier, 1965; Tappan, 1971, p. 1088. Graptobtes: Moore, Lalieker, & Fischer, 1952, p. 726. Fish-Tetroporl ( Crossopterygian-Ampibian ) Transition: Colbert, 1969, p. 71-78; Homer, 1966, p. 72-74, 86-88, 90; Homer, 1968, p. 71-72. Amphibian-Reptile Transition: Colhert, 1969, p. 111114; Homer, 1966, p. 94-96, 102103; Homer, 1968, 1). 86-87, 96. Reptiles: Colhert, 1948, p. 153; Colhert, 1965, p. 170171; Homer, 1968, p. 131, 137, 138. Reptile-Mammal Transition: Beerhower, 1968, p. 477480; Colhert, 1969, p. 130-144, 250, 254; Coffey, 1971a, p. 159; Olson, 1965, p. 40-44, 193-209; Olson, 1971, p. 671731; Homer, 1966, p. 173-174, 178, 186; Homer, 1968, 13. 159, 163-164. Mammals: Colhert, 1969, p. 368-369, 454, 457; Dunhar and Waage, 1969, p. 464; Lull, 1908, p. 180; Lull, 1940, p. 569, 615; MeGrew, 1937, 1). 448; Oshorn, 1929, p. 759, 831; Scott, 1937, p. 335, 476; Stirton, 1959, p. 48; Thomson, 1925, p. 60. |
1971; Crusafont-Pairn & Reguant, 1970). As a result, a casual reader might
conclude erroneously that we see no evolutionary variations within
species. However,
the true situation is that paleontologists frequently ignore such
variation because
it is not pertinent to their immediate goals (Willams, 1953, p. 29), but that
such variation is present as transitional individuals within the
species (Anderson,
1971; Cuff ey, 1967, p. 41, 85-86; Klapper & Ziegler, 1967; Scott
& Collinsnn,
1959; Williams, 1951, p. 87).
Similarly, we also tend to refer transitional fossils to that higher
taxon which
they most resemble or to which their final representatives belong.
Consequently,
the fact that we are dealing with continuously gradatinnal sequences
may he obscured
by our conventional practice of superimposing artificially
disctsntinous, higher-rank
taxonomic boundaries across such lineages (Olson, 1965, p. 100-101,
202-203; Van
Morkhoven, 1962, p. 105, 153; Williams, 1953, p. 29; Cuffey, 1967, p. 38-39).
As a result, for example, in the middle of sequences of transitional
fossils bridging
the conceptual gaps between the various vertebrate classes, we find forms which
sit squarely on the dividing line between these high-rank taxa and which can he
referred to either of two. In addition to Archaeopteryx between
reptiles and birds
(discussed previously), we can also note Diart/trognathus between reptiles and
mammals, the seymouriamnrphs between amphibians and reptiles, and Elpistosiege
between fishes and amphibians (see references in Table 5).
Higher taxa-from genera on up through phylaare useful concepts in handling data
concerning organisms (in fact, they constitute what the layman terms
"major
kinds" of organisms); however, they are artificial mental
constructs rather
than "basic facts of nature" (Brouwer, 1967, P. 161; Olson, 1965, p. 100101,
201-203). Moreover,
although there are reasons why transitional sequences between higher taxa are
not as frequent as we would like (Brouwer, 1967, p. 160-169; Olson,
1965, p. 118,
184-211; Simpson, 1953, p. 366-376; Simpson, 1960, p. 159-161), nevertheless we
can cite some particularly impressive transitional fossils between higher taxa
of various ranks. In addition to those mentioned previously as inter-phylum and
inter-class transitions, others involve higher taxa of class-group rank (Erben,
1966; Raup & Stanley, 1971, p. 306-307), orders (Easton, 1960, p.
446; Miller,
Furnish, & Schindewolf, 1957, p. L22; Teichert, 1964, p. K325),
families (Arkell,
Kummel, & Wright, 1957,
p. L117-119; Brinkmann, 1937; Easton, 1960, p. 425; A Flower, 1941,
p. 526; Moore,
Lalicker, & Fischer, 1952, p. 351), and genera (Arkell, ICummel,
& Wright,
1957, p. L116118; Brinkmann, 1929; Broower, 1967, p. 158; Gimhrede,
1962; Newell,
1942, p. 21, 59; Raup & Stanley, 1971, p. 264).
Evolutionary Implications of Transitional Fossils
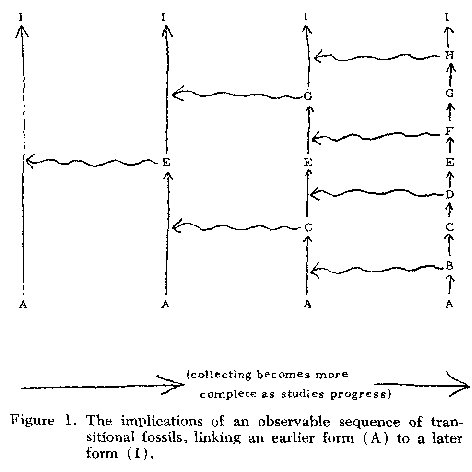
Let us consider the
implications
of an observable sequence of transitional fossils, such as those examples cited
above, linking an earlier form (A, in Figure 1) with a later form
(I). At a preliminary
stage of knowledge, when only the relatively distinct forms A and I are known,
it could be thought (as was actually done in the early 1800's) that the earlier
form (A) had been instantly created, lived for a time, was then eliminated by
some catastrophic environmental event, and after extinction was
replaced by special
creation of the somewhat similar later form (1). As our knowledge of
the paleontologic
record begins to increase, we find a third form (such as E, in Figure 1) which
is morphologically and chronologically intermediate between A and I.
The gap between
A and I is thus partly filled and replaced by two narrower gaps, and
we must invoke
an additional special creation and catastrophic extinction to explain
the observed
record. Continued collecting uncovers more morphologically and chronologically
intermediate specimens (say C and G, and later also B, D, F, and H, in Figure
1); at each step, the new gaps we produce by partly filling existing ones are
progressively smaller, and we must invoke ever more instantaneous creations and
catastrophic extinctions. It is evident that, when we have accumulated a very
large series of transitional fossils grading continuously from A to I
(as we often
now have in the course of population-oriented paleontologic studies), we must
envision a very large number of creations and
catastrophes-approaching, in fact,
the probable number of reproductive generations involved in the
sequence, allowing
for the vagaries of the processes of fossilization and study.
Invoking progressively
more special creations until each generation is interpreted as the
result of special
creation becomes clearly implausible. Instead, noting that many
fossils preserve
ordinary reproductive structures, and also that the differences
between successive
fossil assemblages are of magnitude comparable to those observable
between consecutive
ancestordescendent populations in nature today, we are forced to conclude that
the entire series represents a chain of reproductive generations,
descending one
from the other by the usual natural reproductive processes,
uninterrupted by any
special creative acts from without.
As emphasized above, transitional fossils
are known
between groups of organisms classified at both low and high taxonomic
ranks; i.e.,
between both low- and high-rank taxa.
Low-rank taxa-the many species known to ushave a real existence in nature, in
that they consist of populations or morphologically similar, actually
or potentially
interbreeding individuals which live during a continuous segment of
geologic time.
Transitional fossils between morphologically distinct,
chronologically successive
species require us thus to conclude that a new species results from
the operation
of natural reproductive processes upon successive generations of a population
without the intervention of special creative acts; i.e., through what
the scientist
terms "evolutionary processes".
On the other hand, higher taxa-thosc above speciesrank, from genera up through
phyla-do not have a real existence in nature in quite the same sense
that species
do. Instead, higher taxa of various ranks are simply the scientist's
mental abstractions
by which the many species comprising the organic world are grouped according to
the various degrees of over-all morphologic similarity displayed. Species which
are very similar may be grouped within one genus, while species which have only
a little in common may be grouped together only in the same class or
phylum. Since
higher taxa are no more than aggregations of species, transitional
fossils between
higher taxa indicate simply that, in time, the same natural ancestor-descendent
process producing new species eventually produces a chain of
successive and progressively
more different species, whose final member will be drastically
different in morphology
from its initial member and will therefore be classified by
taxonomists in a different
high-rank taxon. Consequently, the practice has developed among
modern taxonomists
that higher-rank classifications, which are based initially upon
observable degrees
of morphologic similarity among species, also should reflect
evolutionary ancestor-descendent
relationships among those species as much as possible. Moreover, it
also is apparent
that the amount of morphologic change producable by evolutionary processes is
essentially unlimited, given the context of vast eons of geologic time.
As a still broader implication of these considerations, we can define
"evolution"
as the gradual and permanent change in the form and function of adult
living organisms,
of successive generations, over a long period of geologic time. Paleontologic
evidence (discussed here) has played the critical role in developing
this concept,
but numerous other lines of evidence also suggest it. The interested reader can
explore these in other excellent sources (especially Lull, 1940; Olson, 1965;
Simpson, 1953), where he also can learn that the process termed "natural
selection"-far from being carelessly equated to evolution as
some anti-evolutionists
assert-is an important part of the method by which evolution is accomplished.
Moreover, the range in taxonomic ranks over which transitional
fossils are observed
(as described above) shows that what some anti-evolutionists label
"general"
and "special" evolution are merely extreme end-members in the scale of
a single natural phenomenon, evolution, and thus usually do not
warrant separate
consideration.
As defined above, evolution is a scientific (rather than, say, philosophical)
concept, and so comments about the nature of science are relevant here.
Using actual practice as the basis for definition, we can define
"science"
simply as the attempt to understand natural phenomena more completely by means
of repeatable or verifiable observations of natural phenomena. (This is broader
than the rigid, prediction- or experiment oriented definitions developed by some
philosophers not actively engaged in scientific work.) Also, unlike mathematics
or logic, science does not deal in formally rigorous certainties, but instead
strives for conclusions which are at best highly probable. Failure to
understand
this has made extensive, philosophically-based discussions-by
anti-evolutionists,
among others irrelevant. Moreover, while the search for ultimate or first causes
moves into the realm of metaphysics, discussion of possible proximate
or intermediate
causes which might be implied by observational evidence clearly falls
well within
the scope of science.
Still further, we need to realize that there is no fundamental
difference between
what has been termed "historical science" and
"empirical science".
The scholar can be relatively certain of only what he is experiencing
at the present
moment, not of what the objects he is examining imply to him about
the past. This
is as true for the chemist reading his notebook describing
yesterday's experiments
and for the historian examining ancient Egyptian records, as it is
for the paleontologist
viewing the fossils and rock strata which form the pages of a natural textbook.
None of these three can he rigorously certain that their world was
not instantaneously
created minutes ago with all its evidences of apparently longer history (Olson,
1965, p. 49); however, for each, his scholarly interpretations about
events before
the present moment are much more probable than would be purely
conjectural imaginings.
Paleontologists studying sequences of transitional fossils are
clearly operating
in a scientific manner, because their data can be regenerated by anyone willing
to examine the earth's crust independently. As more and more such
sequences come
to light, considering the processes which formed them becomes essential if we
are to understand nature more thoroughly (i.e., still within the
scope of science).
As discussed above, interpreting these sequences as proximately due
to evolutionary
processes becomes ever more probable (in fact, overwhelmingly so, agree all who have been directly involved with the
evidence), while
a fiat creationist interpretation becomes ever less likely. Because of the long
time spans involved, we will never be rigorously certain that our
view is a wholly
accurate reflection of natural reality, but the many transitional fossils known
render evolution already so highly probable that presentation of it
as scientific
fact is quite justified. Finally, as is generally true in the
development of science,
once a concept has been well documented, it can in turn provide a
basis for further
work; the concept of evolution has done just this most fruitfully for
many areas
within the earth and life sciences over the past years.
A few remarks are also appropriate about the theological implications
of evolution
as demonstrated by sequences of transitional fossils. As the reader
may have noted,
theological considerations do not enter at all into our demonstration
of evolution
as a very highly probable scientific conclusion. Consequently, like
other scientific
conclusions, this one cannot be viewed as inherently either pro- or
anti-Christian,
However, of course, Christians-especially theologians-will need to
integrate evolutionary
process into their views as being the proximate means which Cod uses to create
various forms of life, just as He uses other scientifically
demonstrable processes
to maintain the natural universe.
Conclusion
In summary, the paleontologic record displays numerous sequences of
transitional
fossils, oriented appropriately within the independently derivable
geochronologic
time framework, and morphologically and chronologically connecting
earlier species
with later species (often so different that the end-members are classified in
different high-rank taxa). These sequences quite overwhelmingly
support an evolutionary,
rather than a fiat- creation ist, view of the history of life.
Consequently, after
carefully considering the implications of the fossil record, we must conclude
that that record represents the remains of gradually and continuously evolving,
ancestor-descendent lineages, uninterrupted by special creative acts,
and producing
successive different species which eventually become so divergent
from the initial
form that they constitute new major kinds of organisms.
REFERENCES CITED (unedited because of
exhaustion)
Anderson, E.J., 1971, Discrimioaot function analysis of variation
among populations
of the brachiopod Gypirlula coeprsoneosis: Geol. Soc. Amer., Abs. Prog., v. 3,
no. 1, p. 14-15.
Andrews, U.N., Jr., 1961, Studies in Paleohotany; Wiley, New York; 487 p.
Arkell, W,J,, Kummel, B., & Wright, C.W., 1957, Mesozoic
Ammonoidea: p. L80-L465
of Moore, B.C., ed., Treatise on invertebrate Paleontology, pt. L (Mollusea 4,
Ammonoidea), p. L1L490.
Banner, FT., & Blow, W.H., 1959, The classification and
stratigraphical distribution
of the Globigerinaceae: Palaeontology, v. 2, p. 1-27.
Barnard, T., 1963, Evolution in certain biocharacters of selected
Jurassic Lagenidae:
p. 79-92 of von Koenigswald, CUR., ed., Evolutionary Trends in
Foraminifera; Elsevier,
Amsterdam; 355 p.
Barraode, J., 1887, Système Silurien du Centre de la
BohtmeBecheeches Paldootologiqucs;
Praha; v. 7 (Echinodermes), pt. 1 (Cystidtes), 233 p.
Beaver, H.H., 1967, Morphology: p. S300-S344 of Moore, R,C., ed., Treatise on
Invertebrate Paleontology, pt. S (Echinodermata 1), v. 2 (Blastoids),
p. S297-S650.DIALOGUE:
ROGER J. CUFFEY
Beerbower, JR., 1968, Search for the Past-An Introduction to Paleontology, 2nd
ed.; PrenticeHall, Englewood Cliffs; 512 p.
Berggren, WA., 1962, Stratigraphie and taxonomic-phylogenetic studies of Upper
Cretaceous and Paleogene planktonic Foraminifera: Stoekh. Contr. Cool., v. 9,
p. 107-129.
Berry, W.B.N., 1960, Craptolite faunas of the Marathon region, west
Texas: Univ.
Tex. Bur. Eeoo. Geol., P01). 6005, p. 1-179.
Berry, \V.B.N., and Booeot, A.J., 1970, Correlation of the North
American Silurian
rocks: Geol. Soc. Amer., Spec. Pap. 102, p. 1-289.
Bird, SO., 1971, On interpolative open nomenclature: Syst. Zoo]., v.
20, p. 469.
Iloreske, J.R.A., Jr., 1972, Taxonomy and taphonomy of the North American amiid
fishes (abs.): Geol. Soc. Amer., Abs. Prog., v. 4, no. 1, p. 3-4.
Boocot, A.J., & Ehlers, CM., 1963, Two new genera of strieklandid
brachiopods:
Univ. Mich. Mus. Paleont. Contr., v. 18, p. 47-66,
Brinkmann, B., 1929, Statistiselsbiostratigraphisehe Untersuchungen
an mitteljurassisehen
Ammoniten ilber Arthegriff nod Stammesentwieklung: Gesell. Wsss.
(1Ottingen, Ahh.,
math.phys. KI., n. ser., v. 13, no. 3, p. 1-249.
Brinkmann, R., 1937, Biostratigraphie des Leymeriellenstammes nebst Bemerknngen
mr Pallogeographie des Nordwestdeutselien Alb: Geol. Staatsinst.
Hamburg, Mitt.,
v. 16. p. 1-18.
Bronninsann, P., 1950, The genus Ilotstkenino Coshman in Trinidad and Barbados,
BY/I.: Jour. Paleont., v. 24, p. 397-420,
Brouwer, A., 1967, General Paleontology; Univ. Chicago Press,
Chicago; 216 p.
Brown, EM!., 1943, Some prehistoric trees of the United States: Jour. Forestry,
v. 41, p. 861868.
Bulnian, OMB., 1970, Graptolithina, 2nd ed.: p. V1-V163 of Teiehert, C., ed.,
Treatise on Invertebrate Paleontology, 2nd ed., pt. V, p. V1-V163.
Carruthers, E.G., 1910, On the evolution of Zophrentis de
fonouei in Lower Carboniferous times: Geol. Soc. Loud., Quart. Jour., v. 66, p.
523-538.
Chandler, M.E.J., 1923, Geological history of the genus Strotiotea: Geol. Soc.
Loud., Quart. Jour., v. 79, p. 117-138.
Chancy, R.',V., 1949, Evolutionary trends in the angiosperms: p.
190-201 of Jepsen,
CL., Sinipson, CC., & Mayr, E., eds., Genetics, Paleontology and Evolution;
Princeton Univ. Press, Princeton; 474 p.
Charles, R.P., 1949, Essai d'ftude phylogéniqoe des gryphIes liasiques:
Soc. Geol. France, Bull., ser. 5, v. 19, pt. 1.3, p. 31-41.
Charles, R.P., & Manheuge, P.-L., 1952, Les liogryphées du
jurassique
ioffrieor de l'est du bassio parisien: Soc. Geol. France, Bull., ser. 6, v. 1,
pt. 4-6, p. 333-350.
Charles, R.P. & Maohcoge, P.-L., 1953a, Les liogryphIes
jurassi:jues de lest
do bassin parisieo, II, LiogryphIes do Bajocien: Soc. Cool. France, Boll., ser.
6, v. 2, pt 4-6, p. 191195.
Charles, El'., & Maoheoge. P.-L., 1953b, Revision des hogryplsCes do Moser
d'IIistoire Naturehle de Luxembourg: Inst. Grand-Ducal de Lnxesnh., see. set.
nat. phys. math., Arch., n. see., v. 20, p. 183-186.
Cita-Sironi, MB., 1963, Tendanees Cvolutives des foraminitCres
plaoetiqoes (Globotruncanae
) do CrCtacC supCrieur: p. 112-138 of von Koenigswald, G.H.R., ed.,
Evolutionary
Trends 10 Foraminifera; Elsevier, Amsterdam; 355 p.
Clark, 1).L., 1968, Fossils, Paleontology, and Evolution; Brown, Dubuque; 130
p.
Cohhan, WA., 1951, Scaphitoid cephalopods of the Colorado Group: U.
S. Geol. Surv.,
Prof. Pap. 239, p. 1-42.
Cnbbao, WA., 1958, Late Cretaceous fossil zones of the Powder River
Basin, Wyoming
and Montana: Wyo. Geol. Assoc., 13th Ann. Fld. Coof. Cdhk., p. 114-119.
Coithan, WA., 1961, The ammonite family Binneyitidae Beeside in the
western interior
of the United States'. Jour. Paleont., v. 35, 1). 737-758.
Coihan, WA., 1962a, Boeolites from the lower part of the Pierre Shale
and equivalent
rocks in the western interior: jour. Paleont., v. 36, p. 704-718.
Cobban, WA., 1962h, New Boeohites from the Bearpaw Shale and equivalent rocks
of the western interior: Jour. Paleosst., v. 36, 1). 126-135.
Cohban, WA., 1964, The Late Cretaceous cephalopod Horesireros Reeside and its
possible origin: U.S. Geol. Sorv., Prof. Pap. 454-I, p. 11-121.
172
Cnhbao, WA., 1969, The Late Cretaceous ammonites Seop/sites Inn
Reeside and Seophitea
hfppoerepis (DeKay) in the Western Interior of the United States:
U.S. Geol. Sorv.,
Prof. Pap. 619, p. 1.29.
Cobban, WA., & Reeside, J.B., Jr., 1952, Correlation of the
Cretaceous formations
of the western interior of the United States: Geol. Soc. Amer., Bull., v. 63,
p. 10111044.
Coeke, J.M., 1970, Dissepimental rogose corals of Upper Pennsylvanian
(Missoorian)
rocks of Kansas: Univ. Kao. Palennt. Contr., art. 54, p. 1-67.
Colhert, EH., 1948, Evolution of the horned dinosaurs: Evolution, v.
2, p. 145-163.
Colhert, EH., 1965, The Age of Reptiles; Norton, New York; 228 p.
Coihert, E.H.. 1969, Evolution of the Vertebrates, 2nd ed.; Wiley,
New York; 535
p.
Coon, CS., 1962, The Origin of Races; Koopf, New York; 724 p.
Crosafont-Pairo, teL, & Begoant, 5., 1970, The nomenclature of intermediate
forms: Syst. Zooh., v. 19, p. 254-257.
Coffey, R.J., 1967, Bryozoan Tohnfipora eorbooario in Wreford
Megaeyelothem (Lower
Permian) of Kansas: Univ. Kan, Paleont. Contrib., Bryoz. art. 1, p. 1-96.
Coffey, R.J., 1970, Critique of "The Dying of the Giants":
Jour. Amer.
Sri. Affil., v. 22, p. 9396.
Coffey, B.J., 1971a, Evidence for evolution from the fossil record: Jour. Amer.
Sri. Affil., v. 23, p. 158-159.
Coffey, B.J., 1971b, Transitional fossils well known: Jour. Amer. Sri. Affil.,
v. 23, p. 38.
Coffey, R.J., 1972, More on Archaeopteryx: Jour. Amer. Sri, Affil.. v. 24, p.
36.
Deehaseasix, C., 1934, Prineipales espCees de Liogryphfes hiasiqoes,
valeor stratigraphiqoe
et remarques sur qoelr;oes formes motantes: Soc. Geol. France, Boll., ser. 5,
v. 4. on. 1.3, p. 201212.
Donhar, CO., 1963, Trends of evolution in American fusulhses: p. 25-44 of v:m
Kneaigswald, C.H.R., ed., Evolutionary Trends in Foramioifera;
Elsevier, Amsterdam;
355 P.
Doohar, CO., & Rodgers, J.W., 1957, Principles of Stratigraphy; WBey, New
York; 356 p.
Dsmohar, CO., & Waage, KM., 1969, Historical Geology, 3rd ed.; Wiley, New
York; 556 p.
Durham, J.W., 1971, The fossil record and the origin of the Deoternstonsata: N.
Amer. Palenot. Cony,, Proc., pt. H, 1). 1104-1132.
Easton, W.H., 1960, Invertebrate Paleontology; Harper, New York; 701 p.
Elias, M.K., 1937, Stratigraphic significance of some Late Paleozoic feoestrate
bryozoans: Jour. Paleont., v. 11, p. 306-334.
Elias, M.K., 1942, Tertiary prairie grasses and other herbs from the
High Plains:
Geol. Soc. Amer., Spec. Pap. 41, p. 1-176.
Erhen, H.K., 1966, Uber den Ursprong der Ammonoidea: Biol. Rev., v.
41, p. 641-658.
Fay, R.O., 1967, Phylogeny and Evolution: p. S392-S396 of Moore, RU.,
ed., Treatise
on Invertebrate Paleontology, pt. S (Eehioodermata 1), v. 2
(Blastoids), p. 5297.S650.
Fisher, W.L., Rodda, PU., & Dietrieh, J.W., 1964, Evolution of
At/sleto petroso
stork (Eocene, Castropoda) of Texas: Univ. Tex. Bur. Eeon. Geol., Pub. 6413, p.
1.117,
Florin, B., 1951, Evolution in cordaites and conifers: Aeta lhort. Bergiani, v.
15, p. 285-388.
Flower, E.G., 1941, Development of the Mixochoanites: Jour. Paleont., v. 15, p.
523-548.
Flower, RH., & Kummel, B., Jr., 1950, A classification of Naotdoidca: Jour.
Palcont., v. 24, p. 604-616.
Franz, V., 1932, Vieiporus; Morphometrie, Phylogenie nnd Geographic
dee eornpbisehen,
fossilen nod rezcnten Paludioen: Med.-Natorw. Ces. Jena, Denksehr., v. 18.
Franz, V., 1943, Die Cesehiebte der Tiere: p. 219.296 of Heberer, C., ed., Die
Evolution der Orgaoismen; Fischer, Jeoa; 774 p.
Cartoer, S., Jr., 1971, Phylogenetie lineages in the Lower Tertiary eoceolith
genus Chiososohthos: N. Amer. Paleont. Coov., Proc., pt. C, p. 930-957.
Cioihrede, L. de A., 1962, Evolution of the Cretaceous foraosinifer
Kmjp/sojmsjxrs
ebristoeri ( Carsey ) : Jour. Paleont.,
v. 36, p. 1121-1123.
Claessner, M.F., 1960, The fossil decapod Crustacea of New Zealand
and time evolution
of the order Decapoda: N.Z. Cool. Sorv., Paleont. Boll., v. 31, p. 1-63.
Claessner, M.F., 1969, Deeapoda: p. E399-E533 of Moore, E.C., ed., Treatise on
Invertebrate Paleontology, pt. R
JOURNAL OF THE AMERICAN SCIENTIFIC AFFILIATION
PALEONTOLOGIC EVIDENCE AND EVOLUTION
(Arthrnpoda 4), v. 2, p. R399-R651.
Grant, RE., 1962, Trilobite distribution, tipper Franeonia Formation
(Upper Cambrian),
southeastern Minnesota: Jour. Paleont.. v. 36, p. 965-998.
Greiner, 1-I., 1957, "Spinier disjunctus--its evolution and paleoeenlogy
in the Catskill Delta: Yale Univ. Peabody Mus. Nat. Hist., Bull., v.
11, p. 1-75.
Gnber, AL., 1971, Problems of sexual dimorphism, population structure
arid taxonomy
of the Ordovician genus Tetradelta (Ostraeoda): four. Paleont. v. 45,
p. 6-22.
Hall, CA., Jr., 1962, Evolution of the eehinoid genus Asfrodapsis: Univ. Cal.
Pub. Geol. Sei., v. 40, p. 47-180.
Ilanson, ED., 1961, Animal Diversity; Prentice-I-tall, Engiewood
Cliffs; 116 p.
Heaslip, W.G., 1968, Cenozoic evolution of the alticostate venericards in Gulf
and East Coastal North America: Palaeontogr. Amer., v. 6, p. 55-135.
Hottinger, L., 1963, Lee alv€olines paléoglnes, exemple d'un genre
polyphylétiqne: p. 298314 of von Koenigswald, G.H.R., ed., Evolutionary
Trends in Foraminifera; Elsevier, Amsterdam; 355 p.
House, MR., 1970, On the origin of the clymenid ansmonoids: Palaeont., v. 13,
p. 664-676.
Howells, W., 1967, Mankind in the Making, rev. ed.; Doubleday, Garden City; 384
p.
Imlay, R.W., 1959, Succession and speciation of the pelecypod
Aocello: U.S. Cool.
Sorv., Prof. Pap. 314-G, p. 155-169.
Jackson, R.T., 1912, Phylogeny of the Eehini, with a revision of
Paleozoic species:
Boston Soc. Nat. Hi., Men-j., v. 7, p. 1-491.
Jones, D.J., 1956. Introduction to Microfossils; 1-larper, New York; 406 p.
Kauffman, E.G., 1965, Middle and late Turonian oysters of the Lop/ui lugndris
group: Smithson. Misc. Coil., v, 148, no. 6, p. 1-92.
Kanffman, E.G., 1967, Cretaceous T/rrja.riro from the western interior of North
America: Smithson. Misc. Colt., v, 152, no. 1, p. 1-159.
Kauffnsan, E.G., 1969, Form, function, and evolution: p. N129N205 of
Moore, B.C.,
ed., Treatise on Invertebrate Paleontology, pt. N (Mollusea 6, Bivalvia), v. 1,
p. N1-N489.
Kauffman, E.G., 1970, Population systematics, radiometrics and znnation-a new
hiostratigraphy: N. Amer. Paleont. Cony., Proc., pt. F, p. 612-666.
Kaufmaoo, B., 1933, Variations-statistisehe Untersuehungeri 'uber die
'Artahsvandlung"
und "Artunslnldung" an der oberkamhrischeu Trilobitengattuug 0/anus
Dalm: Geol. Pal. Inst. Univ. Greifswrsld, Abh., v. 10, p. 1-54.
Kaufraaun, B., 1935, Exakt-statistisehe Biostratigraphie der Oleoris-Arten von
Sudölaod: Geol. Foren. Stockholm Filrhandl., v. 1935, p. 19-28.
Kay, St., & Colbert, El-I,, 1965, Stratigraphy and Life History; Wiley, New
York; 736 p.
Kermaek, K.A., 1954, A bionsetrical study of Micrresfer caraisguierrem and M.
(Isuoucroster) seuoueissis: Roy. Sue.
Loud., Philos. Trans., ser. B, v. 237, p. 375-428.
Kier, P.M., 1965, Evolutionary trends in Paleozoic echinoidsi Jour. Paleout.,
v. 39, p. 436465.
Kiapper, G., & Ziegler, W., 1967, Evolutionary development of the Icrior/us
fretenicrexcens' group (Couodonta) in the Devonian of Europe and North America:
Palaeuntographiea, ser. A., v, 127, p. 68-83.
Knight, J.B., & Yoehelson, E.L., 1960, Monoplacophora: p. 1771 84 of Moore,
R.C., ed., Treatise on Invertebrate Paleontology, p. I (Mollusca 1), p. I 1-1
351.
Krumbeio, W.C., & Sloss, L.L., 1963, Stratigraphy and
Sedimentation; Freeman,
San Francisco; 660 p.
Kumusel, B., 1970, History of the Earth, 2nd ed.; Freeman, San Francisco; 707
p.
Lang, W.D., 1921-1922, Catalogue of the Fossil Bryuzoa (Polyzoa (-The
Cretaceous
Bryozoa (Polyzoa); British Museum( Natural History), London; v, 3 and 4.
Le Gros Clark, WE., 1964, The Fossil Evidence for Human Evolution,
2ud ed.; Univ.
Chicago Press, Chicago; 201 p.
Lerman, A., 1965, Evolution of Exogpro in the Late Cretaceous of the
southeastern
United States: Jour. Paleoot., v. 39, p. 414-435.
Lewootiu, B.C., 1971, The yahoos ride again: Evolution, v. 25, p. 442.
Lull, B.S., 1908, The evolution of the elephant: Amer. Jour. Sci., ser. 4, v.
25, p. 169-212.
Lull, R.S., 1940, Organic Evolution, rev. ed.; Macmillan, New York; 743 p.
MacNeil, F.S., 1965, Evolution of the genus Myo, and Tertiary
DECEMBER 1972
migrations of Mollusca: U.S. Geol. Surv., Prof. Pap. 483-G, p. G1-G51.
Matthew, W.D., 1910, The phylogeny of the Felidae: Amer. Mus. Nat.
Itist., Bull.,
v. 28, p. 289316.
McGrcw, P.O., 1937, The genus Cprrorctres: Jour. Paleont., v. 11, p.
444-449.
Miller, AK., Furnish, W.M., & Schiodewolf, OH., 1957, Paleozoic Ammonoidea:
p. L11-L79 of Moore, B.C., ed., Treatise on Invertebrate
Paleontology, pt. L (Mullusca
4, Aminonoidea), p. L1L490.
Moore, J.N., l970a, Evolution-required or optional in a science course?: Jour.
Amer. Sci. Affil., v. 22, p. 82-87.
Moore, J.N., 19701), Should Evolution Be Taught?; privately
published, East Lansing;
28 p.
Moore, J.N., 1971a, On chromosomes, mutations, and phy-
lugeny: Amer. Assoc. Adv. Sci., 138th Ann. Mtg., paper,
16 p. (mioreugr.).
Moore, J.N., 1971h, Retrieval system problems with articles in Evolution: Amer.
Inst. Biol. Sci., 22nd Ann. Mtg., Paper 279, 13 p. (mimeogr.).
Moore, J.N., & Slusiser, 11.5., 1971, Biology, A Search for Order
in Complexity;
Zoodcrvan, Grand Rapids,
Moore, B.C., 1959, Protarthropoda: p. 016-020 of Moore, B.C., ed., Treatise on
Invertebrate Paleontology, pt. 0 (Arthropoda 1), p. 01-0560.
Moore, B.C., Lalicker, C. G., & Fischer, AG., 1952, Invertebrate Fossils;
McGraw-Hill, New York; 766 p.
Morris, H.M., 1963. The Twilight of Evolution; Baker, Grand Rapids; 103 p.
Nelson, ES., & Semken, HA. 1970, Paleoecotogical and
stratigraphic significance
of the muskrat in Pleistocene deposits: Cool. Soc. Amer., Bull., v.
81, p. 3733-3738.
Newell, ND., 1937, Late Paleozoic Pelecypods-Pectinacea: Kan. Geol.
Surv., (Psihl.)
v. 10, pt. 1, p. 1-123.
Newell, ND., 1942, Late Paleozoic Pelecypods-Mytilacea:
Kan. Gcol. Surv., (Pohl) v. 10, pt. 2, p. 1-115.
Nichols, D., l959a, Changes in the Chalk heart-urchin Micraster interpreted in
relation to living forms: Boy. Soc. Loud., Plnlus. Trans., ser. B, v. 242, p.
347-437.
Nichols, D., 1959b, Mode of life and taxonomy in irregular sea-urchins: Syst.
Assoc., v. 3, p. 61-80.
Olsoo, E.C., 1965, The Evolution of Life; Mentor, New York; 302 p.
Olson, E.C., 1971, Vertebrate Paleozoology; \Viley-Iutereciemice, New York; 839
p.
Oshmiro, H.F., 1929, The titaisotlseres of ancient Wyoming, Dakota,
and Nebraska:
U.S. Geol. Surv., Stun. 55, p. 1-953.
Papp, A., 1963, Uher die Eutwickluog von Fleterostegioeo: p. 350-355
of von Koenigswald,
G.H.R., ed., Evolutionary Trends in Foramninifera; Elscvier,
Amsterdam; 355 p.
Patterson, B., 1949, Bates of evolution in taemsiodmets : p. 243278 of Jepsen,
G.L., Simpson, C, G., & Mayr, F., eds., Genetics, Paleontology
and Evolution;
Princeton Univ. Press, Princeton; 474 p.
Pokerroy, V., (Transi. Allen, K.A.), 1963, Principles of Zoological
Micrepalaeontology;
Macmillan, New York; 652 p.
Raup, DM., & Stanley, SM., 1971, Principles of Paleontology; Freeman, San
Francisco; 388 p.
Rauzer-Clsernoueova, D.M., 1963, Eimnge Fragco zur Evolution den Fusemlinideen:
p. 45-65 of von Koenigewald, G.H.R., ed., Evolutionary Trends in Foraminifera;
Elsevier, Amsterdam; 355 p.
Rcxroad, C.B., 1958, The conodont homeomorphs Trep/rrogoot/eus and
S(reptugoot/rodus:
Jour. Paleont., v. 32, p. 11581159.
Romer, AS., 1966, Vertebrate Paleontology, 3rd ed., Univ. Chicago
Press, Chicago;
468 p.
Bomer, AS., 1968, Notes and Comments an Vertebrate Paleontology; Univ. Chicago
Press, Chicago; 304 p.
Boss, CA., & Boss, J.P., 1962, Pennsylvanian, Permian rugoee corals, Glass
Mountains, Texas: Jour. Paleout., y. 36, p. 1103-1188.
Bowe, A.W., 1899, An analysis of the genus Microster, as determined
by rigid zonal
collecting from the zone of B/i msc/eemee//a eucieni to that of
Mierosten cor-onguiourn:
Geol. Soc. Lund., Quart, Jour,, v. 55, p. 494-547.
Seagel, R.F., et al., 1965, An Evolutionary Survey of the Plant
Kingdom; Wadsworth,
Belmont; 658 p.
SchilfIe, L., 1929, Ueber Lias uud Doggeradstero: Geol. u. Palaront. Ahh., p.
sen., v. 17, no. 2, p. 1-88.
Scleauh, H., 1963, Ubcr eioige Entwicklungsreiheo you Nuin
eemeBtes und Axsi/fna uad ihre stratigraphisehe Bedeutumsg: p. 282-297 of von
Koenigswald, G.H.B., ed., Evolutionary Trends in Foramaiuifera;
Elscyier, Amsterdam;
355 p.
Scott, A.J., & Collinson, C., 1959, Intraspecific variability in
conodonts-Pobnotolepi.s
glrsbra Ulriels & Bassler: Jour. Paleont., v. 33, 1)• 550-565.
Scott, W.B., 1937, A History of Land Mammals in the Western
Hemisphere, rev. ed.,
American Philosophical Society (repr. Hafuer, New York); 786 p.
Seward, A.C., 1938, The story of the maidenhair tree: Sci. Progr., v.
32, p. 420-440.
Shrock, R.R., & Twenhofel, W.H., 1953, Principles of Invertebrate
Paleontology,
2nd ed.; McGraw-Hill, New York; 816 p.
Simpson, CC., 1951, Horses; Oxford Univ. Press, Oxford; 323 p.
Simpson, CC., 1953, The Major Features of Evolution; Columbia Univ. Press, New
York; 434 p.
Sohl, N.F., 1960, Archeogastropnda, Mesogastropoda, and stratigraphy
of the Ripley,
Owl Creek, and Prairie Bluff Formations: U.S. Geol. Surv., Prof. Pap, 331-A, p.
1-151.
SohI, N.F., 1967, Upper Cretaceous gastropods from the Pierre Shale
at Red Bird,
Wyoming: U.S. Geol. Sorv., Prof. Pap. 393-B, B1-B46.
Sohn, IC., 1962, Stratigraphic significance of the Paleozoic
ostracode genus Corqelhno
Bradfield, 1935: Jour. Paleont., v. 36, p. 1201-1213.
Spath, L.F., 1938, A Catalogue of the Ammonites of the Liassic Family
Liparoceratidae;
British Museum (Natural History), London; 191 p.
Stehhios, CL., Jr., 1949, Rates of evolution in plants; p. 229242 of
Jepsen, CL.,
Simpson, CC., & Mayr, E., eds., Genetics, Paleontology and
Evolution; Princeton
Univ. Press, Princeton; 474 p.
Stenzel, H.B., 1949, Successional speciation in paleontologythe ease
of the oysters
of the sellaeformis stock: Evolution, v. 3, p. 34-50.
Stenrel, H,B., 1971, Oysters: P. N953-N1214 of Moore, R.C.,
ed., Treatise on Invertebrate Paleontology, pt. N (Bivalvia), v. 3 (oysters),
p. N953-N1224.
Stirton, R.A., 1959, Time, Life, and Man-The Fossil Record; Wiley,
New York; 558
p.
Swartz, F.M., 1945, Zonal Ostracoda of the Lower Devonian in New York
and Pennsylvania
(abs.); Geol. Soc. Amer., Bull., v. 56, p. 1204-1205.
Tappao, Fl., 1971, Microplankton, ecological succession and evolution: N. Amer.
Paleont. Cony., Proc., pt. H, p. 1058-1103.
Tedford, RH., 1970, Principles and practices of mammalian
geochronology in North
America: N. Amer. Paleont. Cony., Proc., pt. F, p. 666-703.
Tcichert, C., 1964a, Actiooccratoidea: p. K190-K216 of Moore, R.C.,
ed., Treatise
OD Invertebrate Paleontology, pt. K (Mollosca 3, Nautiloidea), p. K1-K519.
Teichert, C., 1964b, Nautiloidea-Discosorida: p. K320-K342 of Moore, R.C., ed.,
Treatise on Invertebrate Paleontology, pt. K (Mollusca 3,
Nautiloidea), p. K1-K519.
Teilhard de Chardin, P., 1950, Sur un cas remacqoable d'orthogbnbse
de groupe-l'bvolution
des siphnbidbs de Chine: Colloq. Internal. Centre Nat. Rech. Sei., v.
21, p. 169-173.
Thomson, J.A., 1925, Concerning Evolution; Yale Univ. Press, New
Haven; 245 p.
Trevisan, L., 1949, Lineamenti dcll'evoluzinne dcl ceppo di elefanti
eurasiatici
nd Quarternario: La Ricerca Scientifica, v. 19 (suppl.), p. 105-111.
Uzzell, T., & Pilbeam, D., 1971, Phyletic divergence dates of
hominoid primates-a
comparison of fossil and molecular data: Evolution, v. 25, p. 615-635.
Van de Fliert, JR., 1969, Fundamentalism and the fundamentals of geology: Jour.
Amer. Sci. Affil., v. 21, p. 69-81:
Van Morkhoven, F.P.C.M., 1962, Post-Paleozoic Ostracoda; Elsevier, Amsterdam;
204 p.
Wailer, T. R., 1969, The evolution of the Argopecten gibbus
stock (Mollusca: Bivalvia), with emphasis on the Tertiary and
Quaternary species
of eastern North America: Paleont. Soc. Mem. 3, p. 1-125.
Watson, D.M.S., 1949, The evidence afforded by fossil vertebrates on the nature
of evolution: p. 45-63 of Jepsen, CL., Simpson, CC., & Mayr, E.,
eds., Genetics,
Paleontology and Evolution; Princeton Univ. Press, Princeton;
474 p.
Weller, J.M., 1969, The Course of Evolution; McGraw-Hill, New York; 696 p.
Wells, J.W., 1956, Scleractinia: p. F32S-F444 of Moore, R.C.,
ed., Treatise on Invertebrate Paleontology, pt. F (Coelenterata), p.
F1-F498.
Wenger, R., 1957, Die germanisehen Ceratiten: Palaeontogr.,
174
ser. A, v. 108, p. 57-129.
Wilde, C.L., 1971, Phylogeny of Pseudofusulinello and its bearing on
Early Permian
stcattgraphy: Smithsonian Contr. Paleobiol., no. 3, p. 363-379.
Williams, A., 1951, Llandovery brachiopods from Wales with special reference to
the Liandovery district: Geol. Soc.
Moore's Critique of Cuffey's Position
Several comments must be made in critique of Cuffey's position paper.
Within his
very first sentence he contributes to confusion of terminology by
presenting the
alternative: "development or evolution". This suggestion
that development,
during the life time of an organism, is interchangeable with supposed
evolutionary
alteration of one kind of organism into another kind of organism is
the very confusion
that Louis Agassiz and many others in succeeding decades have urged
evolutionists
to avoid. Development of an individual organism and general evolution are not
alternative concepts.
And apparently Cuffey has contented himself with consideration of
physical evidence
from the geological record only; consequently, he has ignored
completely the full
range of data utilized initially by Charles Darwin as he developed
his persuasively
expressed case for imagined changes of species over time. (I assume that Cuffey
realizes the cogency of my explication of the sheerly circumstantial nature of
physical evidence from those areas covered by Darwin.)
Anyway because Cuffey has chosen to concentrate only on the fossil or
paleontological
evidence, and has given his greatest attention to so-called "transitional
fossils", he has limited my task of criticism.
However, before turning to careful examination of his proffered
evidence for so-called
"transitional fossils", a significant lack of understanding
of scientific
methodology on Cuffey's part must be made explicit. He fails to
comprehend evidently
that all empirical work of geologists is confined to what they are
able to study
in their lifetime. That is, most of the actual empirical work of
geologists involves
detection of types of rocks, classification of rock types on or near
the earth's
surface, and examination of material included in rocks (especially sedimentary
rocks), which commonly involves study of inclusions (fossils)
interpreted as parts
of and or impressions of previous living organisms.
Thus his early use of the term "demonstrated" in his second sentence,
and again several times in the Introduction plus many other times in
his position
paper, is ample indication that he does not understand that geologists cannot
demonstrate empirically anything regarding organic evolution which is supposed
to have occurred over time. Geologists can only interpret what they
find as empirical
scientists, as far as the unrepeatable past is concerned, and this fact would
seem to be clearly evident from Cuffey's own words before his last
introductory
paragraph, i. e., "make evolutionary interprepretations
ultimately inescapable".
Of course his evolutionary interpretations are not ultimately inescapable.
Hence, in his zeal to present his ease for "transitional fossils", as
forcefully as he feels he can, Cuff ey fails to realize that all
conclusions that
he offers about "sequences" or "succession" or
"series"
are plainly reconstructions and extrapolations of what geologists
want to interpret
about material found in rocks, after they have first accepted
evolutionary thinking
as a frame of reference. In writing to numerous other geologists about these concepts, I find that they rather reluctantly admit this point; they come
to realize belatedly that the fossil record in no way is sufficient
and necessary
to establish genetic connections between different kinds of
organisms. Absolutely
no known genetic lineage is established from any paleontological
study, no matter
how lengthy the study of the rocks or of the literature about the rocks.
This brings us face to face with another significant shortcoming of
the position
taken by Cuffey. He does not define "evolution" in his introductory
remarks and, when he finally gives attention to such an important point midway
in the section before his conclusion, he leaves his readers in utter confusion.
Cuffey then defines "evolution" in reference to changes in
adult forms
through successive generations. Clearly ambiguous, he does not tell his readers
that he is only addressing his entire line of discourse basically to
changes within
limits of a kind of organism where generation after generation of the same kind
of organism could he extrapolated from the fossil data.
He evidently tries to avoid this restriction on his presentation by referring
to "general" and "special" evolution as extremes "in
the scale of a single natural phenomenon, evolution,..." . But neither he
nor any other geologist can show empirically that the fossils they
find are part
of any "natural phenomenon", as far as illustrating any
genetic lineage
0f one kind of organism with another kind of organism.
His attention to supposed "transitional fossils" is where
Coffey becomes
involved in a blatant ambiguity. He clearly illustrates this fact in his use of
Tables 1 through 5.
All the physical data cited per references included in Table 1 relate solely to
supposed changes of "species within the same higher taxon (genus)".
So in what way can Coffey think that these data are at all relevant
to the question
of explaining change of one kind of organism into another kind of organism? And
the same question can be asked with respect to Table 3 wherein he has
cited referential
materials again of "species within the same higher taxon
(genus)".
It may be true that paleontologists have interpreted
some fossil evidence to involve changes of species within
those kinds of organisms he lists, i.e., angiosperms, foraminiferans,
brachiopods,
gastropods, peleeypods trilobites, and mammals, as far as groups common to both
Tables 1 and 3 are concerned. Nevertheless, paleontologists evidently had no difficulty
in recognizing these kinds of organisms as kinds, and had no basic difficulty
in separating the species of one kind of organism from species of another kind
of organism.
Thus Table 1 and Table 3 are totally irrelevant to any discussion of supposed
changes of one kind of organism into another kind of organism, which
is precisely
the fundamental meaning of organic evolution, as I have made pointedly specific
by affording clear and unambiguous definitions of "general evolution"
versus "special evolution". The evident confusion of the terms with
which Coffey seems to be satisfied is quite clear in his fourth section when he
refers to "evolutionary variations within species". To
juxtapose "evolutionary"
and "variation" in this manner partakes explicitly of
confusion between
supposed changes across limits of kinds of organisms (general
evolution) and those
changes within limits of kinds of organisms (genetic variation, or
microevolution,
if that is what Coffey means), which can be successfully studied in proper
empirical fashion by geneticists.
But to return to Table 2, and then give attention to Tables 4 and 5,
which Coffey
refers to at some length in his section on "examples" of
so-called "transitional
fossils". I again write "socalled" because his
referential citations,
when checked not carefully, do not afford any evidence of change of one kind of
organism into another kind of organism, which is exactly the degree of change
to which Coffey and any paleontologist most address himself, if purporting to
supply physical "evidence" for organic evolution, and not
just limited
changes within boundaries of kind. Space limitations prevent complete, item by
item analysis, but I will give attention to several representative
groups included
in these tables.
For instance, in Table 2, Cuffey cites five sources of information
about hominid
species gradation supposedly "crossing from one higher taxon
into another".
Accepting the clear fact that a "taxon" is essentially
whatever a group
of specialists say it is, then I must point out that proposals about
hominid relationships
by Coon, Howells, Kummel, Le Cros Clark, or Uzzell and Pilbeam are
sheerly conjectural
and speculative because their work is totally devoid of establishment
of any direct
genetic lineage. These men have concentrated on reasoned
extrapolations from the
fossil data, and have offered their speculations about supposed hominid changes
after they have first accepted the thesis of general evolution as I
have defined
it. And the same comment holds for the speculations of E. C. Olson with respect
to supposed reptile to mammal transition included in Table 4.
But most attention should be given to Table 5 because of referential citations
pertaining to three supposed vertebrate transitions: a) fish-tetrapod
(Crossopterygian-amphibian),
b) amphibianreptile, and c) reptile-mammal (also included in Table
4). (Discussion
of supposed vertebrate transitions are always favored by evolutionists.) Here
Cuffey, like most other paleontologists, claims that amphibians have
"evolved"
from fish. However, no one has ever found a single transitional form
showing part
fins and part feet, though these changes would have involved conceivably a vast
multitude of transitional forms.
A certain fish, known as a erossopterygian, is supposed to have
"envolved"
into a labyrinthodont. Noteworthy is the fact that paleontologists reconstruct
the erossopterygian as a fish, equipped with fins, which certainly
did not resemble
a four-footed animal. The labyrintbndnnt, on the other hand, had four feet and
legs according to paleontological reconstruction, and was obviously
an amphibian.
No one would confuse it with a fish.
But no one has ever found a single transitional form
between them! The only reasonable scientific conclusion
seems to be that these transitional forms are not found because they
never existed.
Paleontologists have supposed that a reptile "evolved" into a bird.
Such transition should be traced easily in the fossil record, since forelimbs
of the reptile most have changed slowly and gradually into wings of the bird,
and reptilian scales must have changed slowly also into feathers. However, no
one has ever found a single fossil either with half-way forelimbs and halfway
wings, or with half-way scales and half-way feathers. Nor has any other stage
between reptile and bird ever been found.
Of course, Coffey refers to Archeopteryx as one of the "most famous and spectacular transitional fossils known", as is
so customary with most paleontologists. However, other evolutionists deny this
claim. It is noteworthy that Archeopteryx had claw-like appendages on
the leading
edge of its w'ings; and, a species of birds living today, the
Iloaetzin of South
America, has such claw-like appendages. Also Archeapteryx had teeth, but other
extinct birds, unquestionably 100% birds, had teeth. And though Archeopteryx,
unlike all other birds, had vertebrae extending out along the tail,
nevertheless
Arch eopteryx had 100% wings and 100% feathers.
Thus it is safe to conclude that Archcopteryx was a bird.
Archeopteryx was no more a transitional form between reptile and bird than the
bat is between mammal and bird. An authority on birds has stated:
"The origin
of birds is largely a matter of deduction. There is no fossil of the
stages through
which the remarkable change from reptile to bird was achieved." (Marshall,
A.J., Editor. 1960. Biology and comparative physiology of birds. New
York: Academic
Press, p. 1) (Emphases added) Now this evolutionist did not say that there are
only a few fossils at this supposed transitional stage, but he said there are
no fossils.
And speaking of hats, I would call attention to the cover photograph
of Science,
December 9, 1966, showing a reconstruction of the hones of what is claimed to
he the oldest known bat, and also call attention to comment in the
related article
that no fossil related to a bat had ever been found in the same rocks, or any
older rocks than the claimed age of 50 million years for the bat
bones. Pictured
there was the oldest known bat and it was recognized clearly as 100% bat, the
only mammal that flies, which supposedly "evolved" the
power of flight
over vast lengths of time. Yet no one has ever been able to find a
single fossil
to document this claim.
With reference to supposed transitional forms, the ability to fly
supposedly has
"evolved" separately in four different kinds of animals-the insects,
flying reptiles (pterosaurs), birds, and bats. If general evolution has really
happened, surely we must be able to find some physical evidence in the fossil
record, in at least one or two of these cases. But no evidence can be found for
the imagined evolutionary origin of the ability to fly.
Paleontologist Olson has admitted that as far as flight is concerned there are
some very big gaps in the record (The evolution of life, 1966. New
York: The New
American Library, p. 180). lIe holds that there is almost no information about
the history of the origin of flight in insects. He stated that there
is absolutely
no sign of intermediate stages for the pterosaurs, or flying
reptiles. And referring
to the alleged reptilelike features of Arch copteryx, he had to admit
that Archeopteryx
was definitely a bird with no evidence of presumed evolutionary
ancestors. Finally
he stated that the first evidence of flight in mammals is in fully
developed bats.
Therefore, the fossil record is devoid of any physical evidence for
any imagined
evolutionary origin of flight. There are no transitional forms! (See also Gish,
Duane T. 1972. Evolution: the fossils say no! San Diego: Institute for Creation
Research, 2716 Madison Avenue.)
A further indication of Cuffey's inclination toward lack of precision
in definition
of terms he uses, beyond his perpetrated confusion re the term
"evolution",
is found after his definition of "science" in his words, "there is no fundamental difference between what has been termed
'historical
science' and 'empirical science"'. This is completely false. He
is confused
when he compares the chemist, who actually wrote the notebook he later reads,
and the work of the paleontologist, who never has seen the rocks formed or the
fossils made that he purports to interpret as bases for general evolution.
Even
examination of ancient Egyptian records ranks in a separate category from the
"paleontologist viewing the fossils and rock strata",
because the former
are the products of human effort wherein some Egyptian reported what was
actually seen
or known on a first a
person basis. The paleontologist has no such first-person experience with rocks
or fossils. Contrary to assertions by Coffey, "interpretations
about events
before the present moment", i.e., formation of rock strata and
fossilization
of organisms, are nothing more than "purely conjectural imaginings",
to use his own words.
Evidently Cuffey has been weaving imagined narratives about fossils and rock strata for so long, as have most paleontologists ever since Charles Lyell, a lawyer, made the practice acceptable to the intelligentsia, that Coffey and his colleagues have not come to realize, in any explicit manner, the fact that the whole field of "historical" geology involves a maze of imaginative, speculative narratives as extensive extrapolations into the past. Indisputably, paleontologists are limited only to observational work with rocks, strata, impressions, and inclusions, and such observational work is the extent of their actual empirical scientific work. They cannot repeat events involving such objects. They cannot be scientific by trustworthy, testable, repeatable methods beyond straight forward observation of rocks, strata, impressions, and inclusions. Therefore, all their thoughts about supposed transitional forms, and about imagined past events, are of no value other than as imagined formulations based on circumstantially arranged objects.
When evolutionists, and others probably including Cuffey, refer to such forms as Peripatus and Neopilina as possible transitional forms, or to Jamoytins, Archeopteryx, Seymouria, and Tupaia, as intermediate or linking forms, they merely count on circumstantial similarities which are proposed by the paleontologists in their opinion as evidence to support general evolution. But opinion and speculative, circumstantial interpretations are exactly what the empirical scientist seeks to avoid in preference to conclusive genetic evidence.
The only
true transitional form that could be accepted, it seems to me, is
that form demonstrated
empirically, conclusively as genetically connected to two major kinds
of organisms.
Such conclusive evidence would be obtainable only through cross
breeding experiments
subject to repeatable observations.
Hence, nothing is gained, from
all of Coffey's
careful compilation of referential citations, that counts as physical evidence
for imagined general evolutionary changes of the degree that might
have involved
changes from one type, form, or kind of organism into another type,
form, or kind
of organism. He has provided only data regarding changes supposedly
within kinds
which are essentially to he considered as no more than genetic
variational changes.
Basically, all of his referential citations relate to physical evidence that can be utilized better to
support the concept
of "fixity of kinds". He has failed to provide any true transitional
forms between or across kinds of organisms.