Science
in Christian Perspective
Science
in Christian Perspective Science
in Christian Perspective
Science
in Christian Perspective
From: JASA 11 (March 1959):
2-11.
Rarely has a scientific tool attracted the interest that has been shown toward the radiocarbon method of dating since its discovery some ten years ago. In their journals, the archeologist and geologist are continually presented with time evidence. Where once there were vague time estimates based on such qualitative evidence as sedimentation rates, today a radiocarbon date may appear, expressed to the nearest hundred years. Even the theological journals have recorded the voice of radiocarbon dating as it was called to witness in the case of the Dead Sea Scrolls. In popular magazines and newspapers, too, the atomic clock-as it is often called-is a topic of discussion. It is the purpose of this paper to examine this new aid to chronology. The outline to be followed is as follows: (a) theory, (b) checks on the theory, (c) sample processing and counting, (d) problems, and (e) pertinent dates.
TheoryIn the magazine American Scientist Dr. Willard Libby, now an AEC commissioner and originator of radiocarbon dating, makes this statement,1 "Radiocarbon dating had its origin in the curiosity about the possible effects that cosmic rays might have on the earth and particularly, of course, on the earth's atmosphere. We were interested in testing whether any of the various effects which might be predicted could actually be found and used." One might wonder how the jump from cosmic rays to dating could possibly be made, yet it is the nature of basic scientific study that seemingly down-to-earth applications are its offspring. In Libby's mind there were two facts meshing together. First, Korff had discovered free neutrons in the upper atmosphere, a secondary radiation originating from cosmic ray bombardment of the air molecules; secondly, laboratory work with neutrons had shown that they can transmute nitrogen atoms into the carbon isotope of weight 14, called radiocarbon, an isotope unknown in nature at the time Libby began. Combining these two facts with the obvious knowledge that nitrogen is the atmosphere's most abundant component, Libby concluded that the atmosphere should contain natural radiocarbon.
Next he turned to speculation and assumption. He pictured a newly created carbon-14 atom as readily joining an oxygen molecule to form carbon dioxide.
*Paper presented at the 12th Annual convention of the American scientific Affiliation at Gordon College, August, 1957.
Having postulated the existence of radiocarbon in the dynamic carbon reservoir (i.e., atmosphere, biosphere, and hydrosphere), Libby was next faced with predicting the expected concentration of radiocarbon relative to normal carbon-12 and then devising a method for detecting C14 and measuring its concentration. The radioactive nature of C14 enters all of these problems.
First, if C14 were not radioactive-that is, would not decay to the nitrogen from which it originatedit would have been impossible for Libby to make any prediction about the amount of C14 now in the dynamic reservoir, The reason for this is that the terrestrial amount of C14 would be forever increasing. How long production has gone on no one can say with any certainty. However, laboratory work with artificially made carbon-14 had established it as radioactive with a half-life of about 5500 years. That is, a hypothetical isolated pound of radiocarbon today would be 1/2 pound in 5500 years, 3/4 pound in 11,000 years, 3/8 pound in 16,500 years, etc. Libby realized that if cosmic rays of uniform intensity had bombarded the earth for perhaps the last 100,000 years or so, the present amount of radiocarbon being produced would be balanced by the amount of radiocarbon decaying to the original nitrogen.
As an analogy, consider a funnel into which water is being poured at a constant rate. At first, less water comes out than goes in; but when the level in the funnel builds up to a certain point, it ceases rising and outflow balances inflow. For any particular inflow, there is a corresponding level to, achieve a matching outflow. In fact, one could mathematically predict the equilibrium level from a knowledge of the rate of outflow. Similarly, the laws of radioactivity permit mathematical determination of the quantity of a radioactive isotope if one knows its rate of disintegration. At the time of his speculation, no equipment was in existence for Libby to measure the radiocarbon disintegration rate directly, but he did have a "handle" on the production rate. And the two rates, he had postulated, were the same. That "handle" was the published data on the intensity of the neutrons which father radiocarbon.
The intensity of cosmic-ray-produced neutrons varies in a known manner with both latitude and elevation.2 By integrating the neutron flux over the entire earth surf ace and from the ground up, one can determine how many neutrons per second are available for atomic transmutations. Since laboratory work has shown that neutrons overwhelmingly favor the reaction to produce radiocarbon, there is essentially a one-to-one equivalence between neutron intensity and atoms of radiocarbon produced per unit time. So calculated, Libby's radiocarbon production rate-and disintegration rate as well-is 2.6 C14 atoms per second for each square centimeter of earth surface. The total amount of radiocarbon necessary for such a disintegration rate is 66 metric tons.
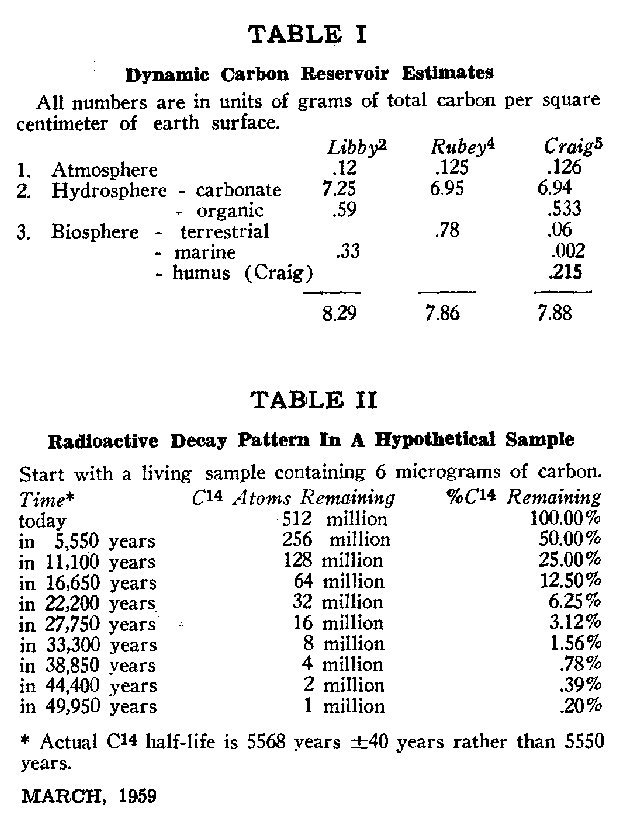
Now to determine the concentration of C14 relative to the total dynamic carbon reservoir requires estimating the size of the reservoir as well. Unfortunate y no convenient "handle" is available such as was the case for the amount of radiocarbon. The three parts of the dynamic reservoir-atmosphere, hydrosphere, and biosphere--must be considered separately. The atmosphere presents little problem since its mass and carbon content are well measured. In the hydrosphere, inorganic carbon, present as dissolved carbonate and bicarbonate, is calculable by methods outlined in Sverdrup's classic text on The Oceans3; the temperature, pH, and so-called alkalinity of the ocean fix the inorganic carbon content within a small range. Dissolved organics are obtained by analysis of many samples. In the case of the biosphere, estimation is more difficult. Libby estimated the amount of biosphere carbon from published annual rates of photosynthesis together with a rough figure of the average carbon atom's residence time in the biosphere. Fortunately, the biosphere is a small fraction of the total dynamic carbon reservoir even in the most liberal estimates, and so any errors in it have little influence on the total estimate. In Table I are listed the reservoir estimates of several men.2.4,5 The controlling size of the hydrosphere contribution and the consistency among estimates are immediately obvious. Libby considers his total to be accurate within +/- 15 percent.
In review, then, before Libby had found any natural
radiocarbon, he had postulated that 66 metric tons of
it were contained within the 42 trillion metric tons of
carbon comprising the dynamic reservoir. If distributed evenly, each radiocarbon atom would
have about
600 billion stable carbon atoms to itself. Should any
portion of the dynamic reservoir-such as a piece of
wood or a clam shell-be cut off from the dynamic
reservoir, as by death, its source of radiocarbon replenishment would also be cut off. Then the radio
carbon concentration would begin to drop through C14
disintegration. Table II shows the decay pattern of
a hypothetical sample containing 512 million radiocarbon atoms at the time of its departure from the dynamic carbon reservoir. In mathematical language, this
table is equivalent to N/N0 = e -693T/T1/2 where No is the
initial number of C14 atoms, N = number of C14 atoms
at time T, and T 1/2
= the C14
half-life of 5568 years.
This is the solution of the basic radioactive decay equation, where X is the decay constant.
It becomes obvious, therefore, how radiocarbon dating is possible. One must first establish the carbon-14
concentration in the dynamic reservoir and then measure the radiocarbon concentration of an object removed from that reservoir. The ratio of the two concentrations is a direct measure of the object's age, provided the reservoir has been uniform in
C14
concentration through time. A ratio of 1/2, for example,
means an age of one half-life or 5568 years.
All this is clear-cut it would seem. But all Libby had then was a potential method of dating. Natural radiocarbon existed only as a necessary conclusion from other facts, and its uniform distribution throughout the dynamic carbon reservoir was merely a postulate. If Libby could detect C14 in the reservoir and could find it disintegrating at about 19 atoms per minute per gram of total carbon, then a new method of dating seemed assured. In short, Libby did detect C14, first in sewage methane that had been enriched in radiocarbon by thermal diffusion, finally in unenriched methane. And the disintegration rate per gram of total carbon was found to be around 15 per minute, amazingly close to the predicted value of 19.
There are a number of empirical checks which can be made on the theory of radiocarbon dating. Five are listed as follows:
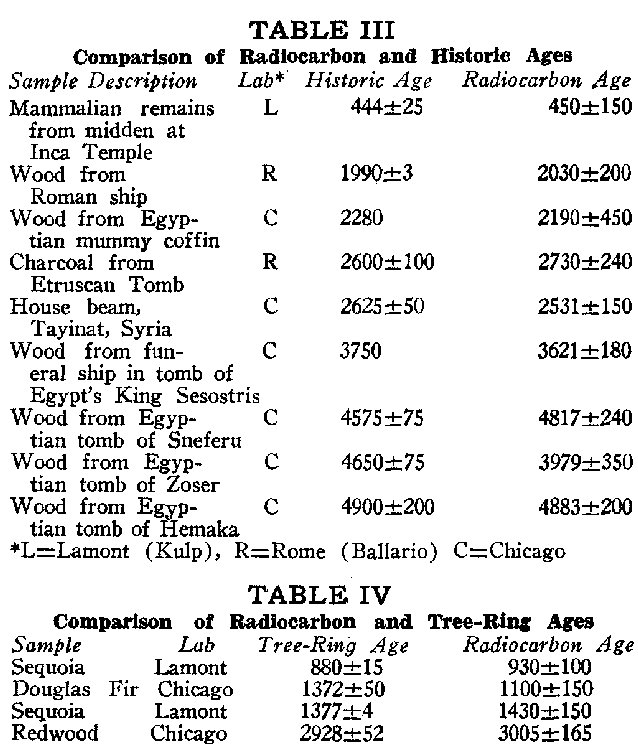
1. Check the uniformity of the dynamic reservoir by measuring the disintegration rates of samples gathered throughout the world.Check No. 2 Tables III and IV list several historical and tree-ring dates along side the corresponding radiocarbon dates.6 Errors attached to the C14 dates are due only to the statistical or random nature of radioactive disintegrations. It is well to emphasize that the dates tabulated are the rule rather than the exception. Unfortunately, historical and tree-ring dates are limited to the last 5000 years or less.
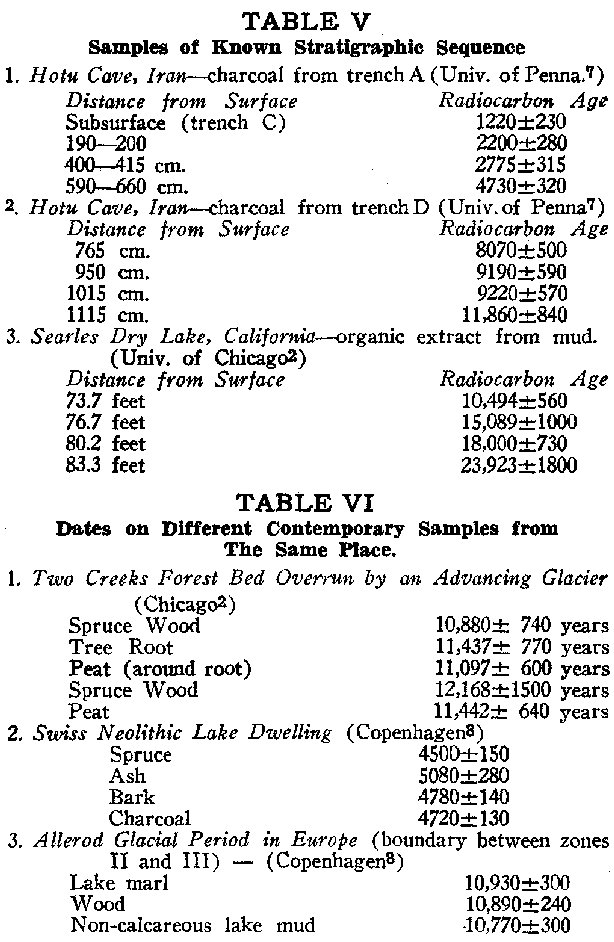
Check No. 3 In Table V are radiocarbon date sequences from an Iranian cave7 and from the bottom deposits of Searles Lake, California.2 Both show the expected progressive age increase with depth.
Check No. 4 Table VI presents dates from three sites each providing several samples that, from field evidence, appeared contemporatieous.2,6,8 Note the different material types dated: peat, marl, charcoal, organic mud, and several different species of wood.
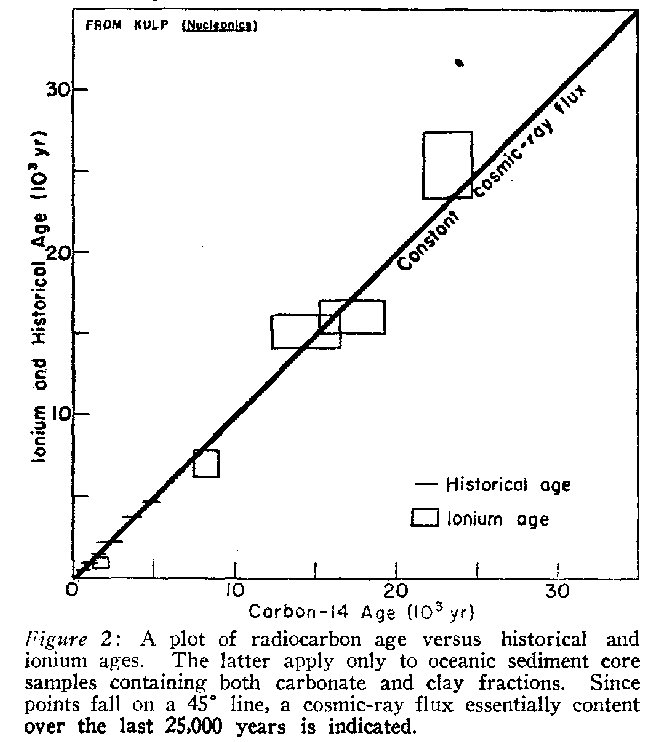
Check No. 5 An independent method for checking C14 dates far beyond historic time is the ioniurn method.9 Limited to certain ocean sediments and independent of cosmic rays, this method is based on the selective adsorption of radioactive ionium (thorium-230) on fine sediments as they settle. The subsequent radioactive decay pattern provides a key to the sediment age. Animal shell fragments included in the sediments provide the carbon for radiocarbon dating. Figure 2 is a plot of ionium ages versus C14 ages for several samples.10 If the two dating methods agree all points fall on a 45 0 line. The use of blocks rather than points is to show the range of error possible in the measurements. This check is significant in that it seems to confirm the assumption that the cosmic ray flux, and thus the rate of radiocarbon production, has been essentially constant for at least 25,000 years.
Another check, not on theory but on technique, is to date a given sample at several laboratories. A number of such checks have been carried out and no significant differences found.
To detect the presence of C14 atoms each with about I trillion surrounding carbon-12 neighbors is obviously a difficult task. Nevertheless, that is the very easiest job required of equipment in a radiocarbon dating laboratory; for one to a trillion is the ratio for the dynamic carbon reservoir. Samples 35,000 years old, for example, have only 1% of this concentration.
No device that measures isotopic concentrations through their mass-such as the mass spectrometer is sensitive enough for natural radiocarbon. Only the fact that the atoms of carbon-14 are continually disintegrating permits concentration measurement. In disintegrating, a radiocarbon atom ejects an electron from its nucleus and becomes a nitrogen atom. This electron is detected-that is, counted-by one of three devices: (a) a Geiger counter utilizing elemental sooty carbon, (b) a scintillation counter with carbon in a liquid compound, and (c) a proportional counter in which carbon is present in gaseous form.
Libby's pioneering work2 was done with a Geiger counter, a cylinder with a wire at its axis. All samples are first converted to C02, the organics by combustion, the carbonates by acidification. The C02 is then reduced to elemental carbon by magnesium metal. After grinding to powdered-sugar fineness, the carbon is spread on the inside of the Geiger counter cylinder. Electrons produced by radiocarbon disintegrations then pass over to the positively charged center wire and trigger a counting device. Today few radiocarbon laboratories employ Libby's black carbon technique, primarily because finely ground carbon is an excellent adsorber of minute airborne radioactive contaminants. These trigger the Geiger counter as readily as radiocarbon.
The second device, a scintillation counter, detects radiocarbon disintegrations through the minute light flashes produced by ejected electrons flying from C14 nuclei. Carbon from the sample to be dated is converted into any one of a number of liquid organic compounds. So as to avoid spurious counts resulting from electronic static, two inter-connected photomultiplier tubes are focused on the sample. Only when both tubes indicate a scintillation is it added to the sample counting register. Were it not for the problems of chemical synthesis, scintillation counting might be standard in radiocarbon laboratories.
The third counting device is the proportional counter utilizing carbon in a gaseous compound. In its construction and operation, the proportional counter much resembles the Geiger counter. Electrons from disintegrating radiocarbon atoms are attracted to a positively charged center wire and thus trigger a counting register. Today, almost all the world's radiocarbon dating laboratories use gas counting, but the particular gaseous compound varies. Some use acetylene, some methane, but most prefer carbon dioxide. At Lamont Geological Observatory, C02 is used.11 It has several advantages: (1) it is produced directly from the combustion of organic samples or the acidification of carbonate samples; hence, no complex chemical syntheses are required, (2) no explosion hazards exist, and (3) the storage of dated samples in the form of solid calcium carbonate is readily carried out. About the only disadvantage of C02 is that it must be ultra-pure in order to count at high efficiency.
Figure 3 is a summary of the counting and processing methods just discussed.On the surface, it would seem that all one need do is to place a sample in a counter for a minute or two, read the counter to get the count rate, ratio this count rate with the rate of a recently living sample, and calculate the age from a simple formula. For two reasons, this idealistic scheme is not possible. In the first place, the time pattern of C14 disintegration is random. One minute 40 counts may be recorded, the next minute perhaps only 20. In the second place, though one may build a tomb about his counter, he still cannot eliminate a certain number of spurious counts called "background."
To solve the first problem, it is necessary only to extend the time of counting. An analogous situation arises when one wishes to prove that a coin should land heads 50% of the time and tails the other 50%. Certainly ten flips will not always show a 50-50 distribution, but the more flips made, the closer to 50/50 will be the overall result. Even so, if a finite number of flips is made, there is a certain statistical error that is present. It is such a statistical error that is indicated by adding -L 100 (or some such number) to radiocarbon dates. At Lamont, every sample is counted at least twice, 16 hours each time. In special cases where one wishes to pinpoint a date, longer times are used. For example, Libby counted one sample almost three months in seeking sufficient accuracy to correlate the Babylonian and Christian calendars.2
The second problem is one of background. When C02 produced from anthracite coal gives a count rate of 15 counts per minute, it is obvious that spurious counts are involved. Coal has been outside the dynamic carbon reservoir so long that no detectable amount of radiocarbon could possibly remain. Then what is the source of the background counts? There are several sources: mesons, gamma radiation, alpha and beta particles.
Of most importance are mesons, highly penetrating particles of cosmic ray origin. Since no practical amount of shielding can cope with mesons, a so-called anti-coincidence ring of about a dozen Geiger counters is placed all around the sample counter. Any meson that passes through the sample counter must necessarily pass through one of the Geiger counters an instant before. By electronic interconnections, it is possible to reject all pulses that occur simultaneously in the sample counter and in any one of the Geiger counters.
Alpha, beta, and gamma radiations originate from minute uranium and other radioactive contaminants that are everywhere present. Iron is comparatively free of such impurities and so the Lamont counters are housed in an eight-inch thick iron tomb. To reduce the background still further, an inch-thick mercury shield envelops the counter, lying inside the anti-coincidence ring of Geiger counters.
With this shielding and electronic circuitry, the background of the Lamont counters is reduced from about 1000 to approximately 15 spurious counts per minute. If this background were to remain constant at all times, there would be no problem. A simple subtraction of 15 from each total count rate would yield the true sample count rate. Unfortunately the background varies somewhat from day to day, apparently correlating with atmospheric pressure change. It is this variation in background together with the statistical uncertainty in its measurement that limits the present dating equipment to about 45,000 years. In other words, when the total count rate of a sample is so low that it approximates the background variation, it is impossible to give the sample a date other than "greater than 45,000 years."At Lamont modern and background samples are counted at least once a week and the electronics are checked daily. Every sample counted is checked for purity. With two counters in operation and everything working properly, about four or five dates can be turned out weekly. Obviously radiocarbon dating is anything but a mass production operation.
ProblemsHow reliable is a given radiocarbon date? It is as reliable as the certainty of two assumptions:
B. that the mass of the dynamic carbon reservoir be
constant with time.
-True if there has been complete isolation from
the dormant carbon reservoir.
From the outline above, assumption 1 depends for its validity upon these physically measurable quantities: cosmic ray intensity, interaction between the dynamic and dormant carbon reservoirs, mixing rates among the parts of the dynamic reservoir, and isotopic ractionation.
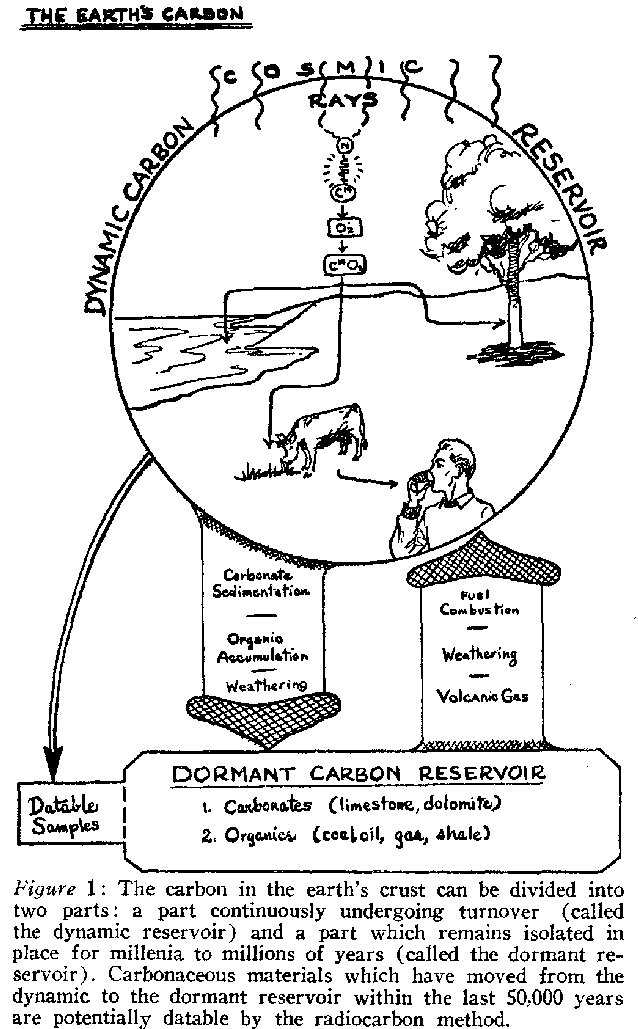
As to variations in cosmic ray intensity over the range of radiocarbon dating, one can offer as data only the checks between lonium and radiocarbon dates mentioned before. The statement of several scientists that there is no known reason for significant cosmic ray variations is of questionable value in discussing the problem.That there is interaction between the dynamic and dormant reservoirs of the earth is obvious. Figure 1 diagramatically shows several ways that carbon-bearing material moves between reservoirs. Carbon with no radioactive content (i.e., dead carbon) enters the dynamic reservoir through the combustion of coal and petroleum, through rock weathering, and through spewed volcanic gases. Carbon leaves the dynamic reservoir as carbonate sediments, organic accumulations such as peat, carbonate weathering products, and of course the samples that are dated. As far as radiocarbon dating is concerned, the question is whether the magnitude of the exchange has been sufficiently great and unbalanced as to have altered the dynamic reservoir appreciably during the last 50,000 years. From the meagre data available the answer seems to be no.
Of all the transfers, the combustion of fossil fuels is apparently the most significant. According to Revelle and Suess,12 "This is probably two orders of magnitude greater than the usual rate of carbon dioxide production from volcanoes, which on the average must be equal to the rate at which silicates are weathered to carbonates." It is estimated13 that almost 4xJ017 grams of combustion C02 have been added to the dynamic reservoir since the modern industrial revolution began. Relative to the total dynamic reservoir, this added carbon is but a fraction of a percent. Relative to the atmosphere, however, it is about 1517o. Almost all of this radiocarbon-free C02 has been introduced since 1900. If it all remained in the atmosphere, trees growing today ought to have a 15% lower C14 concentration than trees of the nineteenth century. Evidence of a lower atmospheric C14 concentration today has indeed been found by Suess14 and others, but nowhere near 15%. Exchange with the vast ocean reservoir has lowered the figure considerably-to about 3% in U. S. industrial areas14 and to about 2% over the earth as a whole.15 Fortunately, this dilution of the dynamic reservoir's carbon-14 content is no problem in radiocarbon dating; it has all taken place so recently. With the advent of nuclear bomb testing man-made radiocarbon has been produced in quantities large enough to more than offset the effect of dilution with combustion carbon dioxide.
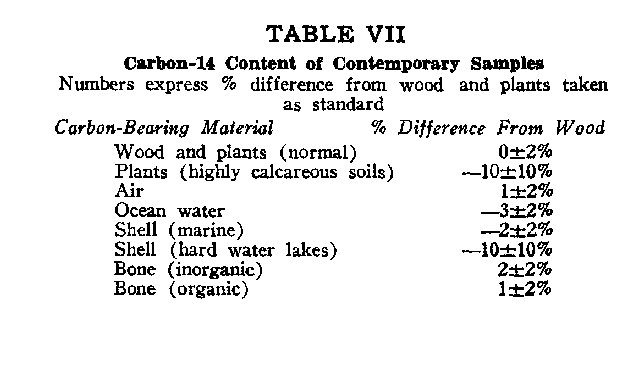
In a way, combustion C02 has been an asset, for it has brought to light information on the rates of mixing between the various dynamic reservoir components.5,12,16 The information so far brought out emphasizes the validity of assuming a grossly uniform radiocarbon distribution throughout the dynamic reservoir; nevertheless, experience reveals some variations. Table VII lists the percent variations from wood taken as the standard of comparison. From this table, it is evident that, in general, the reservoir components pertinent to radiocarbon dating fall within a small range of C14 concentrations. The only exceptions are from unusual environments: hard water lakes17 and caliche soils, both of which provide dead limestone carbon for the organisms they support. Richest of all in radiocarbon is air, partly because C14 originates there but mainly because plants slightly discriminate against utilizing C14 in their life processes.
How then can one utilize the empirical evidence of reservoir variations in radiocarbon dating? At Lamont, wood samples are dated by comparing with the radiocarbon concentration of oak grown in 1890 before the modern industrial revolution. Corrected for radiocarbon lost in the past 67 years, this C14 concentration is assumed to be the same as that of the wood sample when it was alive. A similar technique is used for other types of samples.
In cases where there is opportunity to check historic dates' another scheme is used. Similar material living today is obtained from the area from which the sample came. For example, a recent sample18 was some bread remains from Pompeii, charred by the volcanic ash fall that buried the city in 79 A.D. The estimate of the radiocarbon in this bread when it was still wheat came from wheat growing today near Pompeii. The measured age came within 50 years of the true age.
The second of the two assumptions involved in the reliability of a particular radiocarbon date says simply: the sample is isotopically identical to its living state except for radiocarbon lost by disintegration. Assumption 2 requires:
-Material added through careless field or laboratory handling.
B. that there be no decay or exchange processes whereby one carbon isotope is preferentially enriched or depleted.
Contamination is by far the most frequently heard objection to certain radiocarbon dates. Hunt19 likens the environment of many geologic samples to a laboratory sink and a solution of vinegar. Granting that "sealed test tubes" such as tombs and dry caves should give good radiocarbon dates, he feels that buried samples have little chance of withstanding contamination by fine rootlets or ground water organic solutions.
At Lamont there is constant alert for the contamination of which Hunt and others speak. Unless Lamont personnel collect a sample, it must be assumed that field collection has introduced no contamination. In the laboratory extreme care is taken throughout the sample's journey from glass jar to counting chamber. If surfaces are decayed, outer woodportions are removed; rootlets are picked out carefully; organic materials are acid-leached to remove carbonates, then base-extracted for removal of humic acid.20 Shells are surface cleaned thoroughly, first mechanically and then by acid.To evaluate the significance of contamination, the Lamont laboratory is carrying out a dating prograM20 on geologic samples in the 40,000-year range where even slight contamination is a big problem. By chemically isolating celulose from woods and peats and then comparing the cellulose dates to those of the bulk samples, it should be possible to get a statistical picture of contamination in buried organic materials. The first four samples, two woods and two peats, have indicated no contamination.
Figure 4 shows the age error resulting from various percentages of both contemporary and dead carbon contamination.6 The closer the ages of a sample and its contaminant, the smaller will be the error in the radiocarbon age.
Besides macroscopic contamination, there is the potential problem of atom by atom exchange with the surroundings. That is, carbon-12 atoms in the air or moisture surrounding a sample may interchange with carbon-14 atoms in the sample; or the reverse may happen. For organic samples, this is almost a chemical impossibility. On the other hand, carbonate samples, being ionic, are theoretically capable of undergoing exchange. The evidence available for well preserved shells and calcareous tufa indicates that such exchange is usually insignificant.
Finally, Antevs2 suggested that when a piece of buried wood undergoes decay isotopic fractionation might occur-that is, a larger amount of one carbon isotope might be lost in the decay products, thereby depleting that particular isotope in the sample and thus changing the radiocarbon age. Libby2 examined several contemporary decayed wood samples and was unable to detect such fractionation.
Now to return to the original question: "how reliable is a radiocarbon date?" Because a method's reliability does not hinge on one or even several dates, it is better to reframe the question to read, "how reliable is the radiocarbon method of dating?" Radiocarbon dating is without a doubt theoretically sound and, in the author's opinion, empirically established. Only a small percentage of the several thousand dates so far have been called into question. True, there is still work to be done in the matters of contamination, cosmic ray history, and dynamic reservoir changes. Statistically speaking, however, radiocarbon dating has proved itself.
Without question, there are some radiocarbon dates that are in error. In the opinion of archeologist Frederick Johnson, author of the final chapter in Libby's book on Radiocarbon Dating.2 "barring mistakes by collectors and laboratory workers, the very large majority of the errors are traceable to the process of selection and collection of samples. Such range all the way from faulty observation of conditions in the ground to controversy over the significance of a given stratum."
This points up the fact that this paper is not concerned with the matter of associating a past event with the radiocarbon age of a given sample. The archeological, geological, and anthropological interpretations placed on a sample are completely independent of the very high probability that the sample was alive X years ago. Therefore, one must be cautious in questioning'a radiocarbon date until he has first reviewed the evidence refuting the date. When this involves questioning certain presuppositions in a given field and perhaps revising them, human reluctance to change may sometimes make an objective approach quite difficult. Archeologist Johnson observes,2 "Ininstances where various types of evidence lead to real conclusions concerning chronology, the radiocarbon dates are in general agreement. Where the major difficulty actually appears is in situations where geologists or archeologists do not agree among themselves!In the Appendix are listed a number of published date lists. With dating laboratories springing up all over the world, it will not be long before the total number of dates is measured in tens of thousands; today dates are numbered in the thousands. Starting in May of 1959, most of the world's radiocarbon dates will be published together in a special supplement to be put out annually by Yale University's American Journal of Science.
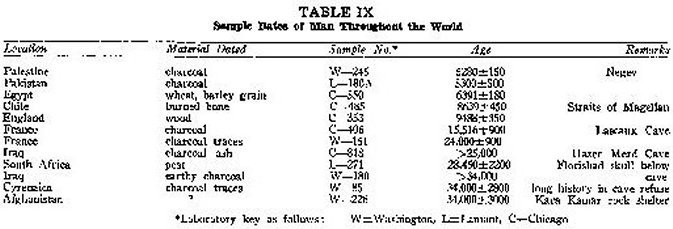
Since this paper is concerned with the age of man, only samples specifically associated with evidences of man's contemporaneous presence are presented. There has been an attempt to pick out the oldest dates of various localities, but this does not guarantee that certain old dates have not been overlooked. The author's field is not archeology, so that in most cases there is no mention of why a sample is thought to date human existence. Generally speaking, the association is through charcoal or burned bone remains in a cave hearth or human relics in a refuse pile. In order to avoid needless detail, ages are rounded off to the nearest hundred years and sometimes to the nearest thousand years. Tables VIII and IX give the specific material dated, the sample number including a letter prefix to identify the laboratory, and the exact published date with its statistical error. More detailed information is to be found in the appropriate date lists; and if these are still inadequate, anyone sufficiently interested can write the archeologists personally.
In the United States until recently, radiocarbon evidence had established man's presence back some 11,000 years. At Fishbone Cave in Nevada, juniper roots and stalks at the base of much human debris had given this date.21 In Texas, Folsom man was fixed around 10,000 years ago2 and Midland man about 7,000 years ago.22 Sandals f rom an Oregon cave gave an age of 9,000 years.2 Nearby Washington gave about the same date.2 From Utah and Missouri22 came dates around 9,800 years, from Nebraska 8,900 years,2 from Alabama 8,000 years,23 and from Tennessee 7,200 years.22
The north central and northeast sections of the United States have shown later dates. For example, the Boyleston Street fishweir in Boston gave one of the earliest New England dates, about 5,000 years.2 This fishweir was unearthed during the digging of a foundation for an office building; it consisted of some 16,000 hand hewn stakes. In New York, charcoal at the base of a refuse pile has dated 5,400 years.2
Within the last couple years, several laboratories have reported dates that seem to triple the antiquity of the oldest American. One such date is from Santa Rosa Island off the northern California coast. A charred bone of a dwarf mammoth was found there with numerous large uncharred pieces of bone from the same animal. The date: 30,000 years.23 From Sandia Cave in New Mexico came a date "greater than 30,000 years" for several mammoth tusk fragments.24 It is Still uncertain, however, whether these fragments establish conteinporaneous human occupation. Perhaps the greatest surprise came within the last year from Texas.
Two charcoal specimens both gave ages greater than 37,000 years.25 Many archeologists question whether this really represents a culture age.
Now a look at several worldwide samples. A date of great interest is that of 8,600 years for burned bone from the tip of South America.2 From France come cave dates of 24,00022 and 16,000 years,2 the latter f rom Lascaux Cave, famed for the wall paintings of ancient animals. England's maximum date2 is around 9,500 years, probably because ice covered the land in previous millenia. Ages beyond 30,000 years come from Iraq,2,26 Cyrenaical 27 and Afghanistan.28 South Africa's Florisbad skull was found beneath a peat layer that dated 28,000 years.21 In general, then, it appears that older ages are more common in Afro-Eurasia than in the Western Hemisphere.
Within the next few years many new dates will be coming out. Perhaps a more detailed picture of man's earthly migrations will then be possible. In the meantime, on the basis of radiocarbon dates alone, it seems reasonable to measure man's tenure on earth in terms of tens of thousands of years.
2. Libby, W. F., 1955, Radiocarbon Dating, 2nd edition: University of Chicago Press, Chicago, Illinois.
3. Sverdrup, H. V., Johnson, M. W., and Fleming R. H., 1942. The Oceans, Prentice-Hall, Inc., Englewood Cliffs, N.J.
4. Rubey, W. W., 1951, Geologic History of Sea Water: Bulletin of the Geologic Society of America, v. 62, p. 11111148.
5. Craig, H., 1957, The natural distribution of carbon dioxide and the exchange time of carbon dioxide between atmosphere and sea: Tellus, v. 9, p. 1-17.
6. Broecker, W. S. , and Kulp, J. L., 1956, The radiocarbon method of age determination: American Antiquity, v. 22, P. 1-11.
7. Ralph, E. K., 1955, University of Pennsylvania radiocarbon dates, 1: Science, v. 121, p. 149-151.
6. Anderson, E. C., Levi, H. and Tauber, H., 1953, Copenhitgen natural radiocarbon measurements, i . Science, v. 118, P. U-11.
9. Volchok, H. L., and Kulp, J. L., 1957, The Ionium metho(l uf age determination; 6eachimica et Cosmuchimica .-icla v. 11, p. 219-246.
10. Kulp, J. L., 1954, Advances in radiocarbon dating: .~ucleumcs, v. 12, p. 19-21.
11. Broecker, VV. S., Tucek, C. S. and Olson, E. A., 1959, Radiocarbon analysis of oceanic C02; Journal of Applied Radiation and Isotopes, in press.
12. Revelle, R. and Suess, H. E., 1957, Carbon dioxide exchange between atmosphere and ocean and the question of an increase in C02 during the past decades: Tellus, v. 9, p. 18-27.
13. Putnam, P. C., 195v, Lnergy in the Futures D. Van Nostrand Co., Inc., Princeton, N. J.
14. Suess, H. E., 1955, Radiocarbon concentration in modern wood: Science, v. 122, p. 415.
15. Eergusson, G. J. 1958, Reduction of atmospheric radiocarbon concentration by fossil fuel carbon dioxide and the mean life of carbon dioxide in the atmosphere: Proceeding of the Royal Society (New Zealand) A, v. 243, p. 561 ff.
16. Arnold, J. R. and Anderson, E. C., 1957, The distribution of C14 in nature; Tellus, v. 9, p. 28-32.
17. Deevey, E. S., Gross, M. S., Hutchinson, G. E., and Kraybill, H. L., 1954, The natural C,, content of materials from hard water lakes: Proceedings of the National Academy of Science, U. S. 40, p. 285-288.
18. Broecker, W. S., Olson, E. A. and Bird, Junius, 1959, Radiocarbon measurements on samples of known age: Nature,19. Hunt, C. B., 1955, Radiocarbon dating in the light of stratigraphy and weathering: Scientific Monthly, v. 81, p. 240247.
20. Olson, E. A. and Broecker, W. S., 1958, Sample contamination and reliability of radiocarbon dates: Transactions of the New York Academy of Science, Series II, v. 20, p. 593604.
21. Broecker, W. S., Kulp, J. L. and Tucek, C. S., 1956, Lamont natural radiocarbon measurements III: Science, v. 124, p. 154-165.
22. Crane, H. R., University of Michigan radiocarbon dates I: Science, v. 124, p. 664-672.
23. Broecker, W. S. and Kulp, J. L., 1957, Lamont natural radiocarbon measurements IV: Science, v. 126, p. 1324-1334.
24. Crane, H. R., 1955, Antiquity of the Sandia culture: Carbon-14 measurements: Science, v. 122, p. 689-690.
25. Brannon, H. R. Jr., Daughtry, A. C., Perry D., Simons, L. H., Whitaker, W. W. and Williams, M., 1957, Humble Oil Co. radiocarbon dates 1: Science, v. 125, p. 147.
26. Rubin, X and Suess, H. E., 1954, U. S. Geological Survey radiocarbon dates 11: Science, v. 121, p. 481-488.
27. Suess, H. E., 1954, U. S. Geological Survey radiocarbon dates 1: Science, v. 120, p. 467-473.
28. Rubin, M. and Suess, H. E., 1956, U. S. Geological
Survey radiocarbon dates III:
Science, v.
123, p. 442-448.
1956, Perspectives:
American Scientist,
.10TIRNAT, (yp THr. AA~MMOAN SOIEN=C APFaJAITON
Radiocarbon Date Lists
Copenhagen
1.
Anderson, E
. C., H. Levi, H. Tauber, Science, July
3,
1953, Vol. 118, pp. 4-6.
11. Tauber, H., Science, Nov.
2, 1956, Vol. 124, pp. 879
881.
Humble Oil Company
1.
Brannon, H
. R., and others, Science, Jan.
25, 1957, Vol.
125, pp. 147.
Lamont Geological Observatory (Columbia University)
1. Kulp, J. L., H. W.
Feely, L
. E. Tryon, Science, Nov.
30 1951, Vol. 114, pp. 565-568.
II. Kulp J. L. and others, Science, Oct.
17, 1952, Vol. 116,
pp. 409-414.
Ill.
Broecker, W
. S., J. L. Kulp, C. S. Tucek, Science,
July
27, 1956, Vol. 124, pp. 154-165.
United States Geological Survey
1. Suess H. E., Science, Sept.
24, 1954, Vol. 120, pp. 467
473.
11.
Rubin, M
and Suess, H. E., Science, April
8, 1955, Vol. 121, pp. 481-488.
111.
Rubin, M
. and Suess, H. E., Science, March
16, 1956, Vol. 123, pp. 442-448.